Ibuprofen is a non-steroidal anti-inflammatory drug that belongs to the propionic acid group.1 Skin reactions to ibuprofen include urticaria, angio-oedema, contact dermatitis and photosensitivity. Fixed drug eruption due to ibuprofen has rarely been described.
Fixed drug eruption is characterised by sudden onset of round and/or oval, oedematous, dusky-red macules on the skin and/or mucous membranes, accompanied by burning and/or itching.2
We report a case of fixed drug eruption due to ibuprofen with tolerance to acetylsalicylic acid.
A 64-year-old man with no history of atopy or drug allergy presented one year ago with three pruritic erythematous macules on his right knee, right calf, and right flank, as well as an aphthous ulcer on the oral mucosa, a few hours after taking allopurinol (Faes Farma SA; Madrid, Spain) and ibuprofen (Kern Pharma SL; Madrid, Spain). The mucosal lesions resolved some days later without treatment, leaving hyperpigmented lesions on the affected skin measuring 4cm in diameter. The patient had previously tolerated both drugs.
The patient was referred to our Allergy Department for further study. He reported that the previous day he had taken ibuprofen and tetrazepam (Sanofi-Synthelabo; Barcelona, Spain) for a muscular contracture. Eight hours after taking the drugs, he developed the same skin lesions, although with no aphthous ulcers on the oral mucosa. Skin sections of the right calf showed a variable dermal perivascular and bandlike lymphocytic and eosinophilic infiltrate with focal basilar vacuolopathy and post inflammatory pigmentation.
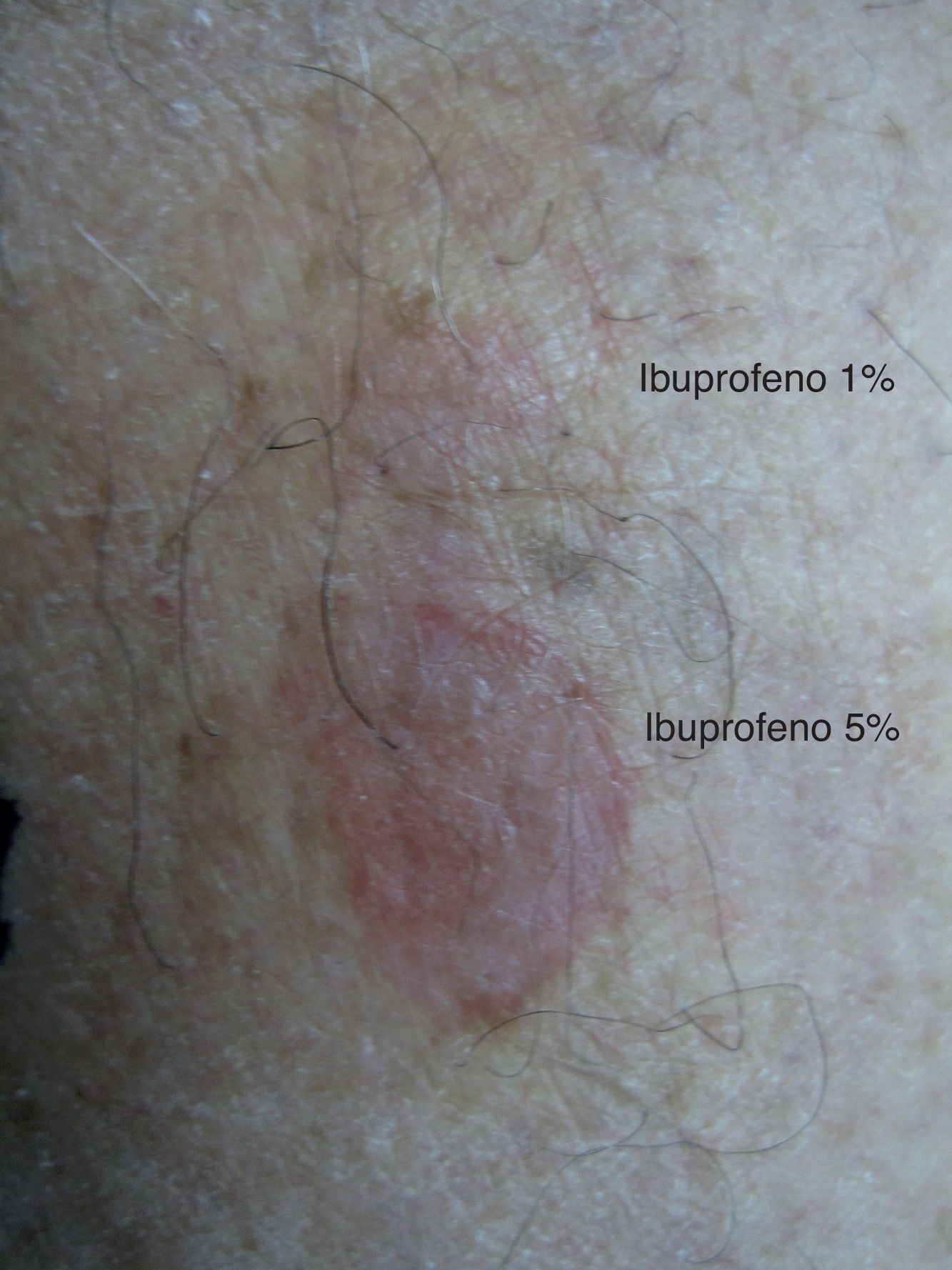
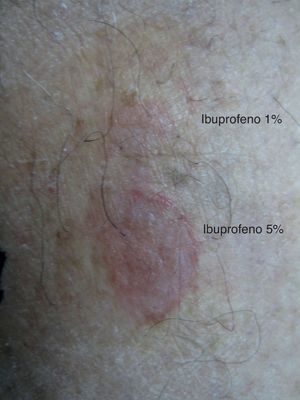
One month later, the patient underwent patch testing (upper back) with allopurinol 1% and 10%, ibuprofen 1% and 5%, and tetrazepam 1% and 10%, all in petrolatum (Nonweven Patch test Strips Curatest®; Lohman&Rauscher International; Rangsdorf, Germany). Ibuprofen 1% and 5% were applied to the calf lesion. The results of the patch test on the residual lesion at 48 and 96h were positive with ibuprofen 1% (++) and ibuprofen 5% (+++) (Fig. 1). The results of the tests on the upper back were all negative. Patch tests in 10 control subjects were all negative.
After obtaining the patient's informed consent, we performed a single blind oral challenge with allopurinol and tetrazepam. The results were negative for both drugs.
To investigate possible cross-reactivity between other non-steroidal anti-inflammatory drugs, we carried out a single blind oral challenge with acetylsalicylic acid, and the result was negative. We therefore recommended the patient to avoid propionic acid group drugs and take only the remaining non-steroidal anti-inflammatory drugs.
Fixed drug eruption is a non-immediate reaction that is well described in the literature.3 The exact pathogenesis of fixed drug eruption is unknown.2
Patch testing is a simple and safe method to identify certain causative agents of fixed drug eruption, especially if residual lesions persist.2 In our case, patch test with ibuprofen was positive in a residual lesion, while patch test with the same drug was negative in upper back.
We were curious to know if our patient was sensitised to the rest of the propionic acid group, but due to the small area affected skin, we could not test these additional drugs. Therefore all propionic acid group drugs were forbidden. The patient tolerated acetylsalicylic acid, and then we recommended the use of the rest of non-steroidal anti-inflammatory drugs.
Díaz-Jara et al.1 described two children, one of them with a fixed drug eruption after the ingestion of ibuprofen. Patch test was positive with ibuprofen on the residual lesion. Kanwar et al.4 analysed 98 cases of fixed drug eruption and in only six of these ibuprofen was the responsible drug. The diagnosis was performed by provocation tests.
Kuligowski et al.5 described a patient with a multiple fixed drug eruption with mucosa affectation due to ibuprofen. Patch test was positive on affected skin.
In summary, we report a case of fixed drug eruption due to ibuprofen that was confirmed by positive patch test and skin biopsy. The patient tolerated acetylsalicylic acid, therefore all propionic acid group drugs were forbidden.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors have no conflict of interests to declare.