Rheumatoid arthritis (RA) is a chronic and systemic autoimmune disease capable of causing substantial joint damage and disability. Anti-TNF therapeutic agents, such as etanercept, infliximab, and adalimumab, have been reported to be effective treatment alternatives in RA patients who are not well-controlled by conventional anti-rheumatic drugs.1,2 Both immediate and delayed type hypersensitivity reactions have been described with etanercept and infliximab since they are considered immunogenic.3,4 However, the administration of adalimumab, as a fully humanised anti-TNF-α, has not been expected to cause immune mediated reactions but it has been associated with both a variety of dermatological reactions, including injection site reaction (ISR) or systemic hypersensitivity reactions.3,5 We describe a patient who presented with progressive ISR to adalimumab (Humira®) injections, with positive skin test and successful subcutaneous rapid desensitisation.
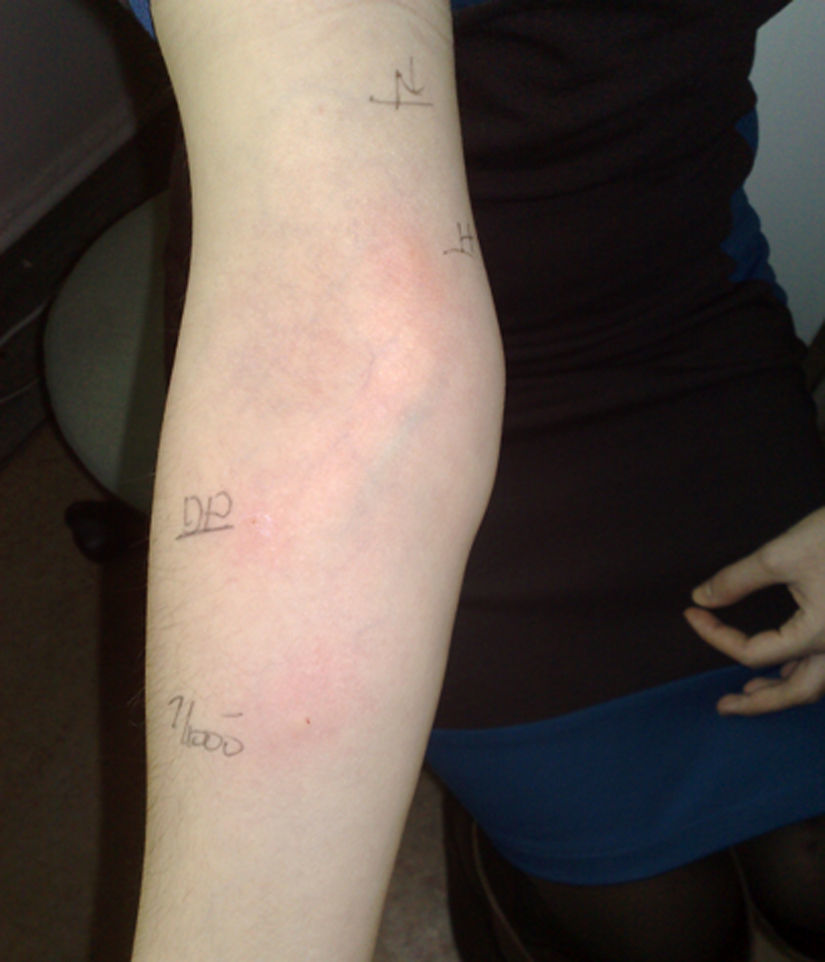
A 26-year-old woman had an eight-year history of RA, having received hydroxychloroquine, oral steroid, methotrexate, and sulfasalazine without significant benefit and was switched to 100mg of infliximab, infusion twice monthly. She responded very well to infliximab, but experienced a decrease in the efficacy of the drug after seven years. She was then started on 40mg/0.8ml adalimumab subcutaneous injections, twice monthly, with significant improvement. She tolerated injections for five months and then developed pruritus, redness, and swelling 4cm×4cm in diameter at the site of injection within 1hour of the 11th injection. The redness and swelling lasted 4–5 days before gradual resolution. A second and third injection of adalimumab again resulted in pruritus, redness, and swelling 8cm×8cm and 8×10 in diameter, respectively, at the site of injection. The timing of the appearance and resolution of these reactions mimicked the first reaction. She denied any systemic reaction. Prick testing with adalimumab (50mg/ml) was negative but intradermal testing with 1/1000 dilution resulted in a 6mm×6mm wheal, along with a 20mm×25mm flare and no late reactions (histamine control was 6mm×8mm along with 17mm×20mm flare) (Fig. 1). Five healthy controls were negative on intradermal testing to this dilution.
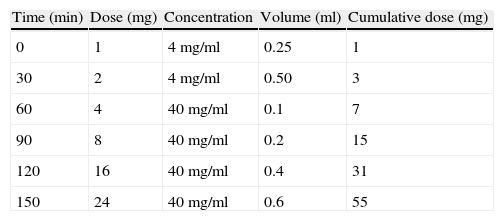
Given the success of adalimumab and the lack of response to other drugs, a six-step rapid desensitisation protocol was generated as shown in Table 1. Informed consent was obtained from the patient before desensitisation and resuscitation equipment was kept ready at the bedside. Oral Benadryl 25mg×1 and famotidine 20mg×1 were given 30minutes prior to the desensitisation. The patient received initially doubling doses starting at 1mg of adalimumab and ending at 24mg of the drug and the cumulative dose for the desensitisation was 55mg. Subsequently the patient received 40mg at each treatment, which is the recommended therapeutic dose. The initial dose was slightly higher, to generate pharmacokinetic blood levels that could sustain the desensitisation. During desensitisation, the patient reported local pruritus, redness, and a swelling 8cm×8cm in diameter within 4–5hours after the last dose of the drug; for this reason, twice-daily Zyrtec was also added on the day of desensitisation. The desensitisation protocol was administered in the inpatient department for the first two injections. The patient was maintained on weekly adalimumab self-injection for three months with Benadryl 25mg and twice-daily Zyrtec. Since she experienced no reactions for the first three months, the adalimumab injections were spaced out to every other week. She now reports mild redness and small local reactions, which are 2cm×3cm in diameter, at the injection site that resolve within one day. As a complementary evaluation, the patient was re-tested nine months after the procedure, and she still reacted to 1/100 dilution intradermally with 6mm×6mm wheal and 20mm×20mm erythema, indicating continued sensitisation with clinical symptoms suppressed at the time of the desensitisation, and two controls were negative at 1/100 dilution, indicating the lack of non-specific reactivity.
Injection site reactions are common clinical presentations of subcutaneous administered anti-TNF drugs. Studies about immunological mechanisms, which underlie injection site reactions, are few and far-between and the immune mechanism underlying ISRs to anti-TNF drugs continue to be unresolved.6,7 Most ISRs are thought to be an example of T lymphocyte-mediated delayed type hypersensitivity reactions6; however, the underlying mechanism may be TH2-mediated and continued treatment may not always be advisable.7 In keeping with this, skin tests have been found positive in some patients with ISRs to etanercept.3 There are only two reports assessing the immunological mechanism of ISRs to adalimumab.3,8 In one of them, intradermal tests with adalimumab were only positive at late reading, with a negative in the prick test suggesting a cell-mediated reaction in two cases of ISRs to adalimumab.3 In contrast, the other study, for the first time, suggested an IgE-mediated immediate type hypersensitivity by demonstrating positivity on skin prick, an early reading intradermal test, and histamine release assay in two patients with progressively worsening ISRs as each subsequent injection of adalimumab resulted in a more prominent oedema and erythema around the injection site which developed more rapidly.8 Similarly, our patient showed progressively more severe ISR and positivity at the immediate reading of the intradermal test, supporting an immediate and possible IgE-mediated immune reaction. Desensitisation is a promising method for the delivery of monoclonal antibodies after a moderate or severe immediate type hypersensitivity reaction in which there is no suitable alternative.4 Recently, desensitisation to ISR, for the first time, was reported by our group in a patient with ISR to etanercept.9 Desensitisation to adalimumab after a systemic reaction such as generalised urticaria, rhinitis, and anaphylaxis has been previously reported in just two case reports,5,10 but desensitisation to ISR to adalimumab has never been reported and, to our knowledge, our case is the first report of subcutaneous desensitisation with adalimumab for ISR.
In conclusion, subcutaneous rapid desensitisation may be a valid alternative for patients with IgE-mediated ISR to adalimumab.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentRight to privacy and informed consent. The authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.