Adverse drug reactions are a frequent cause of consultation in allergology and dermatology services that affect up to 10% of the population (20% in hospitalised patients). More than 10% of them are hypersensitivity reactions. Fixed drug eruptions (FDE) are defined as erythematous plaques recurring at the same anatomical localisation in every occasion that the drug is re-administered to the allergic patient. Lesions are well delimited rounded or oval plaques, erythematous to violaceous with an erythematous border, pruriginous and painful, various centimetres in diameter, and which can evolve to vesicles and leave residual hyperpigmentation.
Together with the exanthemas, urticaria, angio-oedema and multiforme erythema, FDE are the most common allergic reactions to drugs, constituting about 10% of all drug reactions.1,2 The drugs most commonly involved in FDE induction include phenolphthalein, sulphonamides, barbiturics, tetracyclins, aspirin and phenylbutazone.2 In the present paper we will discuss the case of a patient who developed FDE to cross-reacting drugs containing the imidazole ring.
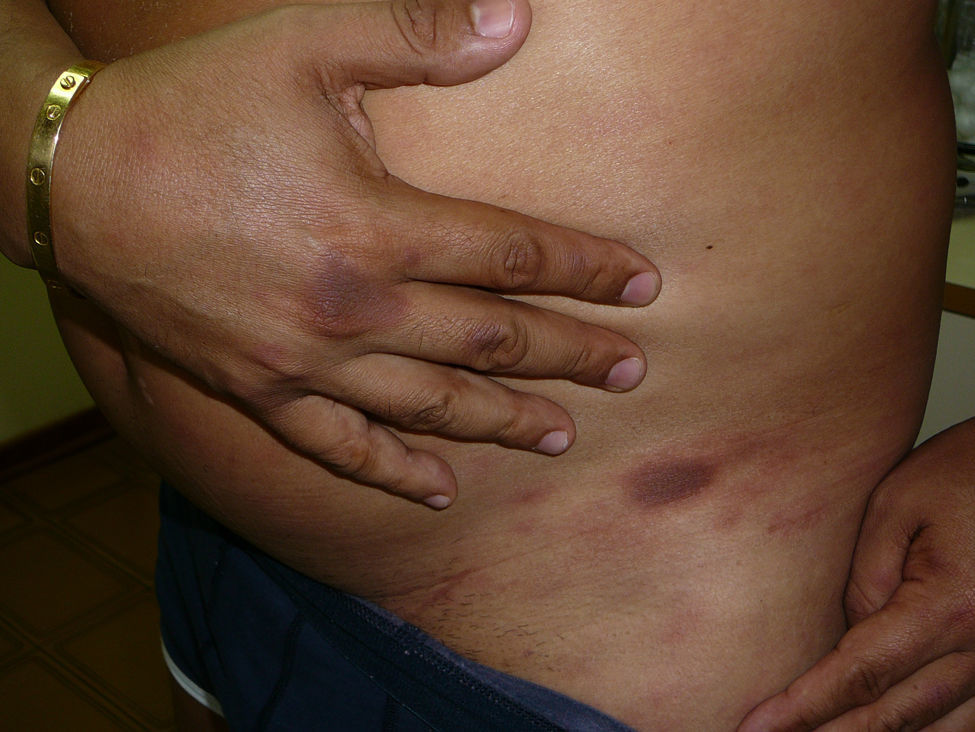
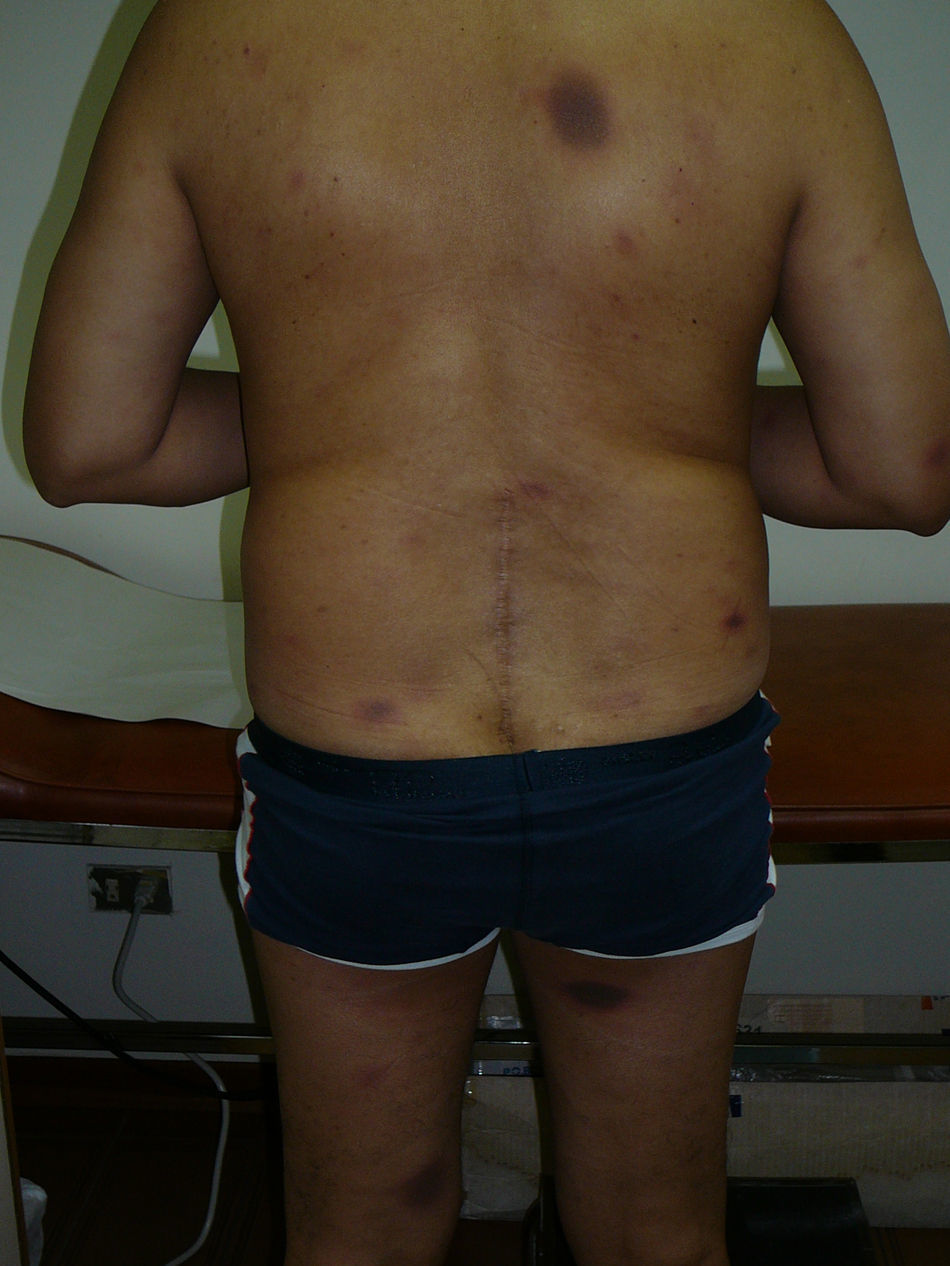
A 51-year-old male patient consulted because he noticed recurrent rounded erythematous and hyperpigmented plaques in the same anatomic areas for the last five years. The lesions were localised in the back, posterior surfaces of the thighs, dorsal aspect of the right hand, and lumbar region. The patient referred that he had similar lesions at the same sites in multiple occasions every time he took self-prescribed metronidazole for non-specific gastrointestinal complaints such as abdominal pain and meteorism. At the time he consulted to the allergy clinics he had lesions with the same characteristics as described above that had appeared four days after starting treatment with oral ketoconazole, 200mg/d, which was prescribed for tinea pedis (Figures 1 and 2).
This patient has a FDE caused by two drugs that contain the imidazole ring in their chemical structure, metronidazole and ketoconazole. This nucleus is present in various frequently used, mainly antifungal and anti-parasitic pharmaceutical products (Table 1). Cross-reactions between imidazolic compounds, clinically manifested as FDE, have been rarely reported in the literature. Cross-reactions appearing as FDE have been observed between metronidazole and tinidazole,3,4 and between metronidazole and albendazole.5
Drugs that contain the imidazole ring
| Action | Drugs |
| Anti-fungal | Bifonazole, Clomidazole, Clotrimazole, Croconazole, Econazole, Fenticonazole, Ketoconazole, Isoconazole, Miconazole, Neticonazole, Oxiconazole, Sertaconazole, Sulconazole, Tioconazole |
| Anti-parasitic | Metronidazole, Tinidazole, Albendazole, Secnidazole |
Contact dermatitis due to cross-reactions between tioconazole and other imidazoles,6 and between ketoconazole and miconazole7 have also been published. Therefore, cross-reactions secondary to the topical application of imidazole-containing drugs can occur. Other cross-reactions of the imidazolic drugs occur with the preservative isothiazolonone. We are not aware of any published report of cross-reactivity between metronidazole and ketoconazole.
FDE due to cross-reacting drugs have been also observed between non-steroidal anti-inflammatory drugs of the oxicam group (piroxicam, tenoxicam, droxicam), and between oxyphenbutazone and phenylbutazone.8
Although this patient did not have any underlying condition that might predispose him for FDE, it has been proposed that diseases that are accompanied by intense immune stimulation, such as autoimmune and infectious diseases (for example, HIV and other viral infections), can increase the susceptibility to develop allergic drug reactions, especially those mediated by T cells (type IV allergic reactions).
The diagnosis of FDE is generally done by means of a history of drug exposure and examination of the lesions. The drug aetiology can be confirmed through a provocation test (contraindicated in patients with severe reactions) and by means of patch testing.8 In our case the patient had induced himself the skin lesions repeatedly during five years, and the diagnosis was so clear that a confirmatory challenge or patch tests were not considered necessary. The patient was instructed to avoid all imidazolic drugs, and a list of medications that contain the imidazole ring was provided. After avoidance for three years, no new skin lesions have appeared.