Peanut allergies are common and can be life-threating for sensitised individuals. Peanut allergens share significant amino acid homology with those of other legumes and tree nuts, but their cross-reactivity still remains unclear.
ObjectiveWe sought to determine the clinical significance of the cross-reactivity of peanut allergens with those of walnut and soybean.
MethodsPooled sera from eight subjects with both peanut and walnut specific IgE were investigated in an inhibition test. After the sera were incubated with either peanut or walnut protein extracts, the quantity of IgE antibodies against the peanut and walnut was measured using an immunoCAP test. Likewise, pooled sera from 18 subjects with both peanut and soybean specific IgE antibodies were incubated with either peanut or soybean protein extracts and evaluated with a peanut and soybean immunoCAP test. SDS-PAGE and immunoblotting were also performed with peanut, walnut and soybean protein extracts and relevant sera.
ResultsPeanut specific IgE was inhibited up to 20% and 26% by walnut and soybean protein extracts, respectively. In reverse, walnut and soybean specific IgE were inhibited up to 21% and 23% by peanut protein extracts, respectively. In the immunoblot analysis, pooled serum from the subjects with peanut specific IgE antibodies reacted with walnut protein extracts significantly.
ConclusionAlthough the clinical significance of the cross-reactivity of peanut specific IgE with walnut and soybean protein extracts has not been established, we believe that individuals who are allergic to peanuts need to be cautious about consuming walnuts and soybeans.
The prevalence of food allergies has been increasing in recent years and can affect up to 10% of young children and 2–3% of adults globally.1,2 Food allergies can cause urticaria, angio-oedema, rhinitis, wheezing, and in some cases can be life threatening due to anaphylactic shock. A recent meta-analysis study reported that fatal food anaphylaxis has an incidence rate of 1.81per million person-years.3
Peanut is one of the primary allergenic foods. It contains at least 18 proteins that have been shown to cause specific IgE reactivity and have a higher potential to cause anaphylactic shock compared to other foods.4–6 The peanut allergens, vicilin (Ara h 1), 2S albumin (Ara h 2), and 11S globulin (Ara h 3), share significant amino acid homology with their respective allergens from other legumes and tree nuts.7–9
Tree nut, despite its botanical taxonomic distance, is believed to be cross-reactive with peanut, due to the high number of individuals reported to be co-sensitised or co-allergic to peanut and tree nut.10 A study of the prevalence of peanut and tree nut allergies among 1218 newborns in the United Kingdom revealed that 1 in 200 children could have adverse reactions to either peanuts or tree nuts by the age of four.11 In another study among individuals with nut allergies, 65% reacted specifically to peanuts of which 30% also reacted with other tree nuts.12
Soybean, belonging to the same legume family as the peanut, has also been investigated as a cross-reactive food with peanuts. Of 69 legume-sensitive individuals, 43% showed sensitivity to soybean and 87% to peanut.13 It has also been reported that soybean immunotherapy in a peanut allergy mouse model resulted in a significant reduction of clinical symptoms following peanut challenge compared with placebo-treated mice.14
However, despite the above findings, the clinical significance of cross-reactivity between peanut allergens and those of walnut and soybean remains unclear. With this in mind, we sought to determine the cross-reactivity of peanut allergens with those of walnut and soybean in the sera of allergic patients.
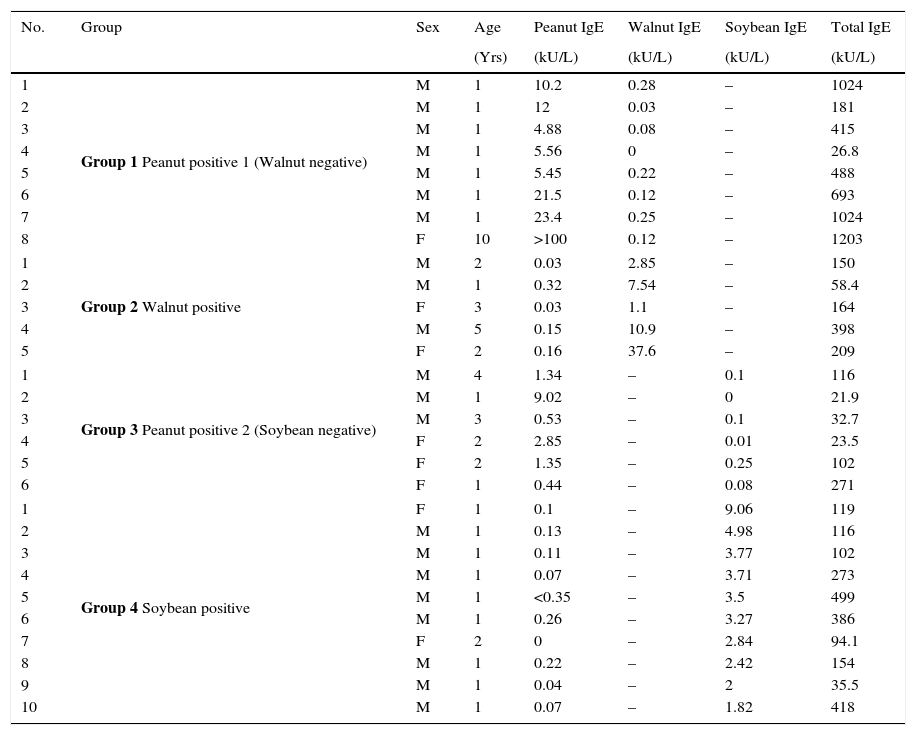
Materials and methodsSubjectsPooled sera from eight subjects with allergic sensitisation to both peanut and walnut were used to investigate cross-reactivity of peanut and walnut in an inhibition test (Supplemental Table 1). For the peanut and soybean cross-reactivity test, the pooled sera from 18 subjects with both peanut and soybean specific IgE antibodies were evaluated (Supplemental Table 2). Allergic sensitisation, the presence of specific IgE, was evaluated using an immunoCAP test (Phadia AB, Upsala, Sweden). The clinical data of the subjects used for the immunoblot analysis are summarised in Table 1. The pooled sera from eight subjects without an allergic sensitisation were used as a normal control. All sera were obtained from Severance Children's Hospital of Yonsei University, Seoul (South Korea). This study was approved by the institutional Review Board of Severance Children's Hospital of Yonsei University.
Characteristics of the subjects from whom sera was used for immunoblot analysis.
| No. | Group | Sex | Age | Peanut IgE | Walnut IgE | Soybean IgE | Total IgE |
|---|---|---|---|---|---|---|---|
| (Yrs) | (kU/L) | (kU/L) | (kU/L) | (kU/L) | |||
| 1 | Group 1 Peanut positive 1 (Walnut negative) | M | 1 | 10.2 | 0.28 | – | 1024 |
| 2 | M | 1 | 12 | 0.03 | – | 181 | |
| 3 | M | 1 | 4.88 | 0.08 | – | 415 | |
| 4 | M | 1 | 5.56 | 0 | – | 26.8 | |
| 5 | M | 1 | 5.45 | 0.22 | – | 488 | |
| 6 | M | 1 | 21.5 | 0.12 | – | 693 | |
| 7 | M | 1 | 23.4 | 0.25 | – | 1024 | |
| 8 | F | 10 | >100 | 0.12 | – | 1203 | |
| 1 | Group 2 Walnut positive | M | 2 | 0.03 | 2.85 | – | 150 |
| 2 | M | 1 | 0.32 | 7.54 | – | 58.4 | |
| 3 | F | 3 | 0.03 | 1.1 | – | 164 | |
| 4 | M | 5 | 0.15 | 10.9 | – | 398 | |
| 5 | F | 2 | 0.16 | 37.6 | – | 209 | |
| 1 | Group 3 Peanut positive 2 (Soybean negative) | M | 4 | 1.34 | – | 0.1 | 116 |
| 2 | M | 1 | 9.02 | – | 0 | 21.9 | |
| 3 | M | 3 | 0.53 | – | 0.1 | 32.7 | |
| 4 | F | 2 | 2.85 | – | 0.01 | 23.5 | |
| 5 | F | 2 | 1.35 | – | 0.25 | 102 | |
| 6 | F | 1 | 0.44 | – | 0.08 | 271 | |
| 1 | Group 4 Soybean positive | F | 1 | 0.1 | – | 9.06 | 119 |
| 2 | M | 1 | 0.13 | – | 4.98 | 116 | |
| 3 | M | 1 | 0.11 | – | 3.77 | 102 | |
| 4 | M | 1 | 0.07 | – | 3.71 | 273 | |
| 5 | M | 1 | <0.35 | – | 3.5 | 499 | |
| 6 | M | 1 | 0.26 | – | 3.27 | 386 | |
| 7 | F | 2 | 0 | – | 2.84 | 94.1 | |
| 8 | M | 1 | 0.22 | – | 2.42 | 154 | |
| 9 | M | 1 | 0.04 | – | 2 | 35.5 | |
| 10 | M | 1 | 0.07 | – | 1.82 | 418 | |
To obtain protein extracts, peanuts, walnuts and soybeans were purchased and finely ground. The ground meals were defatted with cold (4°C) acetone (1:5, w/v) under constant stirring for one hour and then filtered with filter paper. The defatting procedure was repeated until the filtrate became clear. The defatted meals were completely air dried at ambient temperature and then stirred in phosphate buffered saline (PBS, pH 7.4) (1:4, w:v) for 48h at 4°C. The extracts were centrifuged twice at 13,000×g for 30min at 4°C. The supernatants were filtered and dialysed against several changes of distilled water for 48h at 4°C. The extracts were centrifuged once again, lyophilised, and stored at −20°C until use.
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE)The extracts were resuspended in PBS and the protein concentration of each of the extracts was measured by the Bradford assay. Equal concentration of the samples was mixed with loading buffer (60mM Tris–HCl, 25% glycerol, 2% SDS, 14.4mM 2-mercaptoethanol, 0.1% bromophenol blue) and heated for 5min in a heating block at 4°C. The samples were then analysed by SDS-PAGE. Following electrophoresis, the gels were stained with Coomassie brilliant blue or used for immunoblotting.
IgE immunoblottingProteins separated by SDS-PAGE were electrotransfered onto a polyvinyl-difluoride membrane. After blocking with 5% skimmed milk in PBS containing 0.1% tween 20, immunodetection of IgE-binding proteins was performed by sequential incubation with subjects’ sera (diluted 1:10) and alkaline phosphatase-conjugated goat anti-human IgE (¿-chain specific) (Sigma Chemical, MO, USA). IgE reactive protein bands were visualised using 5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt/nitro-blue tetrazolium chloride (Promega, USA).
Inhibition testPooled sera, which were positive for both peanut and walnut by ImmunoCAP, were incubated with 0, 0.1, 1, 5, 10, 50, 100μg of either peanut or walnut protein extracts (9:1, w:v) over night at 4°C. The IgE-reactivity of the inhibited sera against peanut and walnut was then measured by immunoCAP. Pooled sera of both peanut and soybean ImmunoCAP positive sera were also inhibited with either peanut or soybean protein and measured by a CAP radio allergosorbent test against peanut and soybean. House dust mite extract (Dermatophagoides pteronyssinus; Yonsei University, Seoul, Korea) was used as a negative control inhibitor.
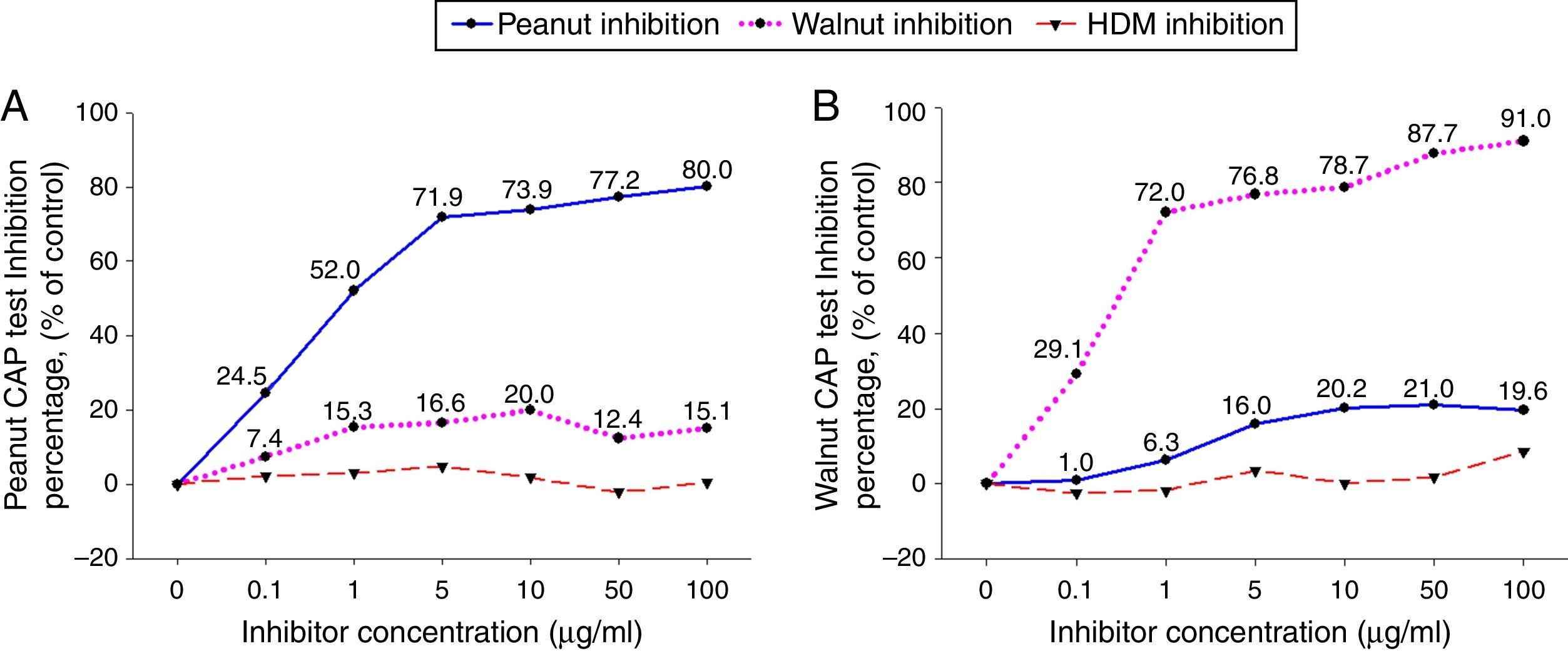
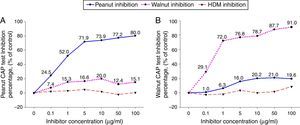
ResultsCross-inhibition of peanut with walnut and soybeanTo observe the cross-reactivity between peanut and walnut, pooled sera from eight subjects who were immunoCAP positive for both peanut and walnut (sex: male 6, female 2, age: 3.1±3.0yrs, peanut specific IgE level of uninhibited pooled serum: 8.57[kU/L], walnut specific IgE level of uninhibited pooled serum: 8.32[kU/L]) were incubated with protein extracts from either peanut or walnut. The sera were then evaluated for specific IgE activities using a peanut and walnut immunoCAP test. As shown in Fig. 1, peanut specific IgE levels were inhibited up to 80% by peanut protein extracts in a dose dependent manner, while only 20% was inhibited by walnut protein extracts. Walnut specific IgE levels showed similar tendencies and were inhibited up to 91% by walnut protein extracts but only 21% by peanut protein extracts.
Cross-inhibition of peanut with walnut. Pooled sera from eight subjects who were immunoCAP positive for both peanut and walnut (>3.66kU/L) were incubated with the indicated concentration of either peanut or walnut protein extracts at 4°C overnight. House dust mite extract was also incubated with the pooled sera as a negative control. The IgE levels of the inhibited sera against peanut (A) and walnut (B) were then measured by the immunoCAP test. The specific IgE level of uninhibited pooled serum was used as a control.
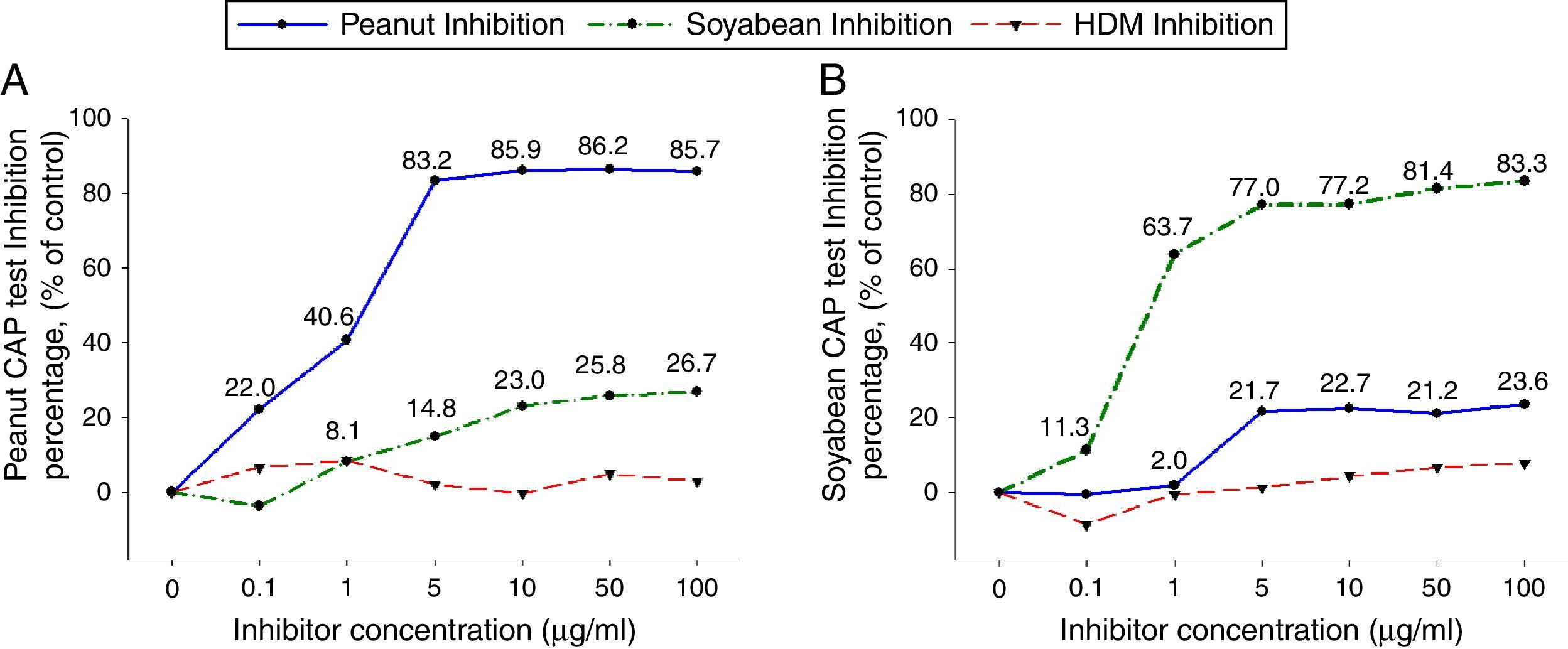
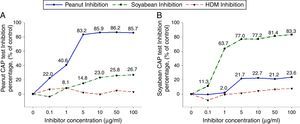
To investigate peanut and soybean cross-reactivity, pooled sera from 18 subjects who were immunoCAP positive for both peanut and soybean (sex: male 16, female 2, age: 3.3±3.4yrs, peanut specific IgE level of uninhibited pooled serum: 31.8[kU/L], soybean specific IgE level of uninhibited pooled serum: 20.3[kU/L]) were incubated with either peanut or soybean protein extracts as described above. Then, an immunoCAP test for peanut and walnut specific IgE was conducted with the inhibited sera. Peanut specific IgE activity was inhibited up to 86% at an inhibitor concentration of 50μg/ml by peanut protein extracts, while only 26% was inhibited by soybean protein extracts (Fig. 2A). Soybean specific IgE also showed similar pattern inhibited up to 83% by soybean protein extracts but only 23% by peanut protein extracts (Fig. 2B).
Cross-inhibition of peanut with soybean. Pooled sera from 18 individuals that were immunoCAP positive for both peanut and walnut (>3.62kU/L) were incubated with the indicated concentration of either peanut or soybean protein extracts at 4°C overnight. House dust mite extract was used as a negative control. Then, the IgE levels of the inhibited sera against peanut (A) and soybean (B) were measured by the immunoCAP test. The specific IgE level of uninhibited pooled sera was used as a control.
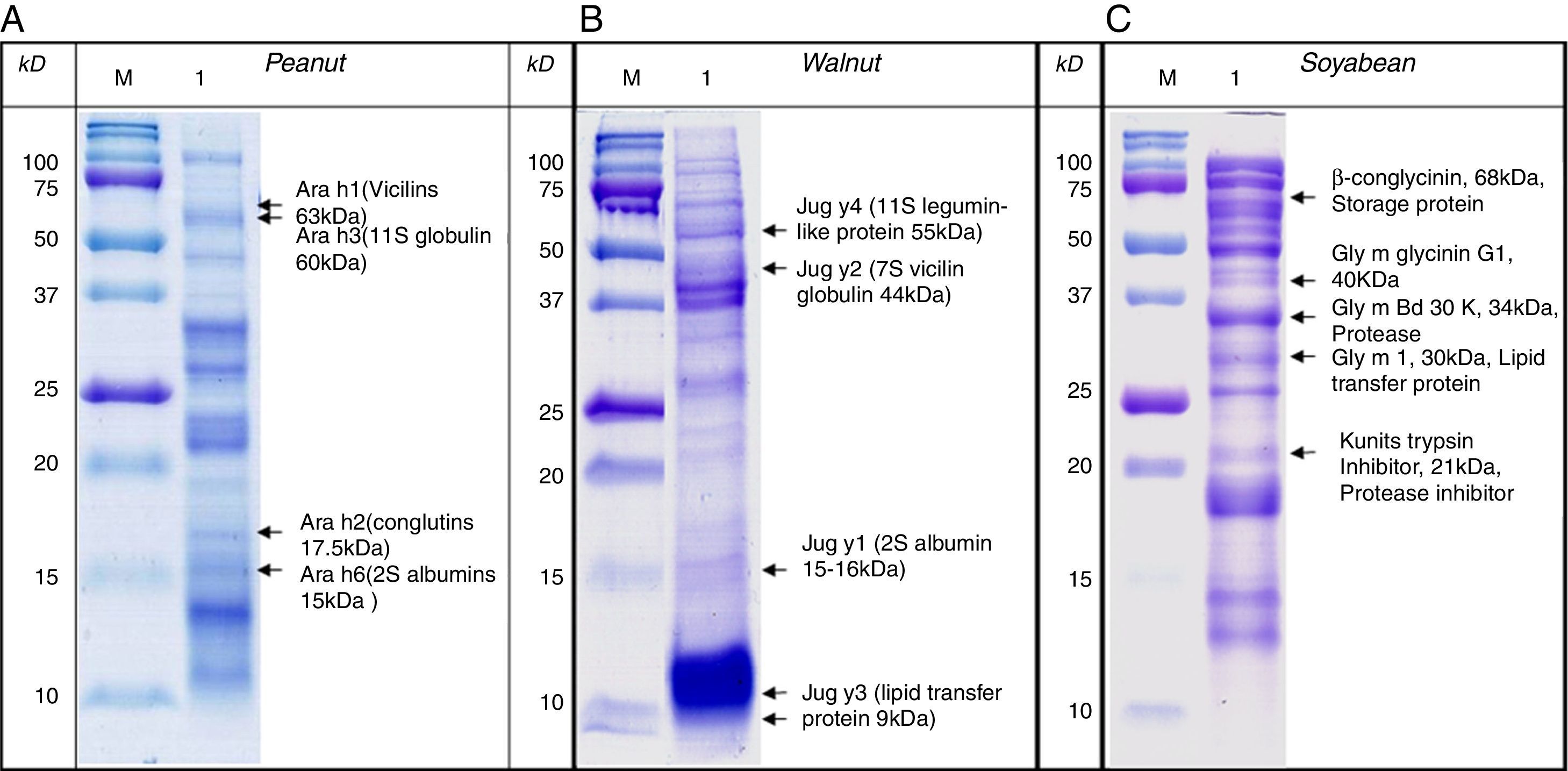
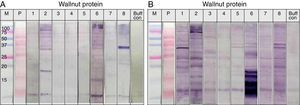
SDS-PAGE was conducted with protein extracts from peanuts, walnuts, and soybeans to observe the distribution of constitutive proteins (Fig. 3). This was followed by immunoblotting with various sera to ascertain which specific peptides were responsible for IgE reactivity and to determine whether the pooled sera positive for either peanut, walnut, or soybean reacted with corresponding allergens from each other (Table 1, Fig. 4).
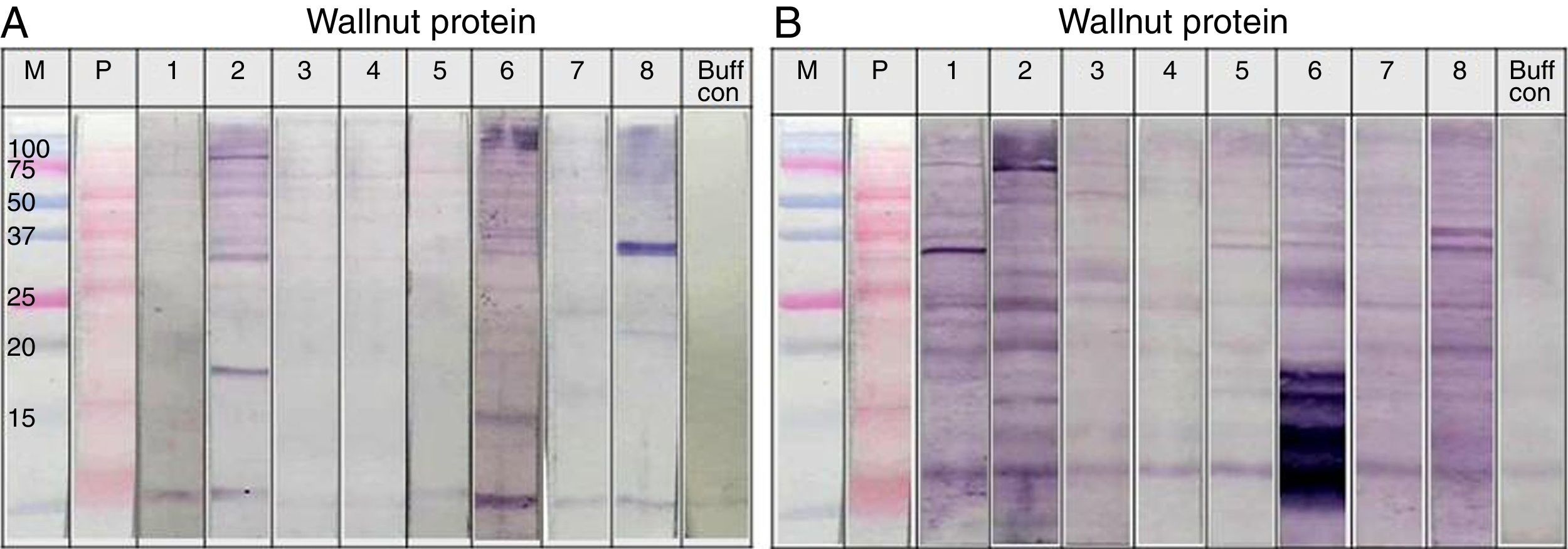
Immunoblot analysis of peanut (A), walnut (B) and soybean (C) protein extracts with pooled sera each containing peanut, walnut and soybean specific IgE. M: Molecular weight marker, PN 1: pooled sera with peanut specific IgE without walnut sensitisation (Table 1. Group1), WN: pooled sera with walnut specific IgE without peanut sensitisation (Table 1. Group 2), PN 2: pooled sera with peanut specific IgE without soybean sensitisation (Table 1. Group 3), Soy: pooled sera with soybean specific IgE without peanut sensitisation (Table 1. Group 4), Normal: pooled sera without allergic sensitisation, Buff Con: buffer control.
As shown in Fig. 4A, lane 2, pooled serum of the peanut immunoCAP positive (>0.35kU/L) sera reacted strongly with peanut protein extracts as we expected. Pooled serum of each walnut and soybean immunoCAP positive sera also showed weak IgE reactive bands to peanut protein extracts. However, this binding was probably non-specific as a similar pattern was seen in the membrane with the normal control serum which is from the subjects without an allergic sensitisation (Fig. 4A).
Again, as we expected, pooled serum of walnut imunoCAP positive (>0.35kU/L) sera reacted with walnut protein extracts (Fig. 4B, lane 2). Interestingly, peanut immunoCAP positive serum also showed a significant reactive band with walnut protein extracts (Fig. 4B, lane 3). Even though several bands were visible on the membrane using the normal control serum, the peanut specific IgE positive serum produced a stronger and different pattern of reactive bands especially between 20 and 60kDa with walnut protein (Fig. 4B).
Pooled sera of soybean immunoCAP test positive sera also bound well to soybean proteins while peanut immunoCAP test positive serum showed non-specific bands similar to the control sera (Fig. 4C). Of the soybean proteins, high molecular weight peptides reacted more strongly than low molecular peptides particularly between 60 and 70kDa.
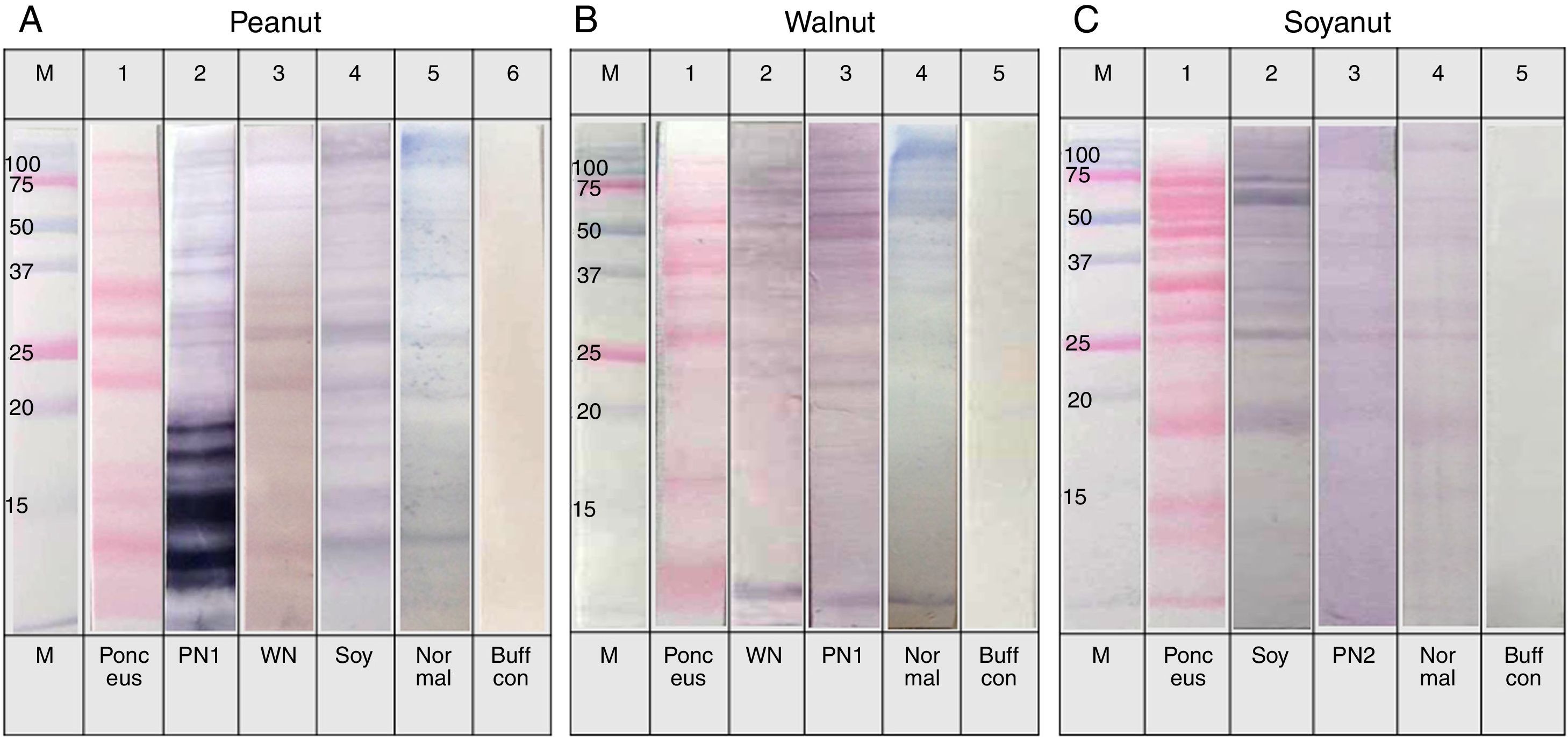
Individual sera immunoblot analysisThe sera showed reactive IgE band to walnut protein in Fig. 4B, lane 3, even though the sera were positive for peanut but not to walnut by the immunoCAP test. To confirm whether the sera could react to walnut protein, an immunoblot analysis was repeated using individual sera. As shown in Fig. 5, significant IgE reactive bands were seen in three of the eight individual sera (Fig. 5A. lanes 2, 6 and 8). Each cross-reacting serum revealed a different IgE band pattern binding to walnut proteins of different sizes. However, each of the three sera bound to a peptide of around 30kDa. In addition, significant cross-reactive IgE bands were observed around 15kDa in lanes 2 and 6.
Immunoblot analysis of walnut (A) and peanut (B) protein extracts using peanut specific IgE positive individual sera (Table 1. Group 1).
The cross-reactivity of peanut with walnut has been studied because they share IgE binding epitopes structurally related to the vicilin allergen in addition to having some structural homology to legumin allergens. Indeed, avoidance or restricted consumption of walnut has been recommended for peanut-sensitised individuals.8,15 In this study, our results from the inhibition test showed only 20% inhibition of peanut specific IgE by walnut protein extracts. This level of inhibition seems low, but when immunoblots of walnut protein extracts were performed with group 1 individual serum that were positive for peanut but negative to walnut by immunoCAP test, three out of the eight subjects showed significantly reactive IgE bands. We were careful to distinguish whether the vicilin and legumin peptides were reactive in both the peanut and walnut protein extracts, but this was difficult to confirm based on identification of the peptides by molecular weight alone. However, IgE reactive bands around 30kDa were seen in all of the three reactive subjects, and one of them was particularly strong (Fig. 5A, lane 8). This peptide is not known as a major allergen of walnut; therefore, further investigation is required for its identification and characterisation. Furthermore, IgE reactive bands were also seen around 15kDa, a similar molecular weight to Jug r 1, in two of the three reactive sera (Fig. 5A, lane 2, 6). As Jug r 1 (14–16kDa) and Jug r 3 (9kDa) are known to be associated with systemic reactions, it is recommended that even though the result of the immunoCAP test for walnut is negative, individuals with peanut allergies should be cautious about ingesting walnuts.16–20
Soybeans are used widely in various foods for both humans and animals. Peanut Ara h 3 has been reported to share IgE epitopes with the glycinin G1 acidic chain of soybean. A study with the sera of individuals allergic to both soybean and peanut showed that the absorption of soybean-binding antibodies by antigen-affinity chromatography resulted in a 73% reduction of IgE binding to peanut allergens.21,22 In our results, although the inhibition of peanut specific IgE by soybean protein extracts was higher than by walnut protein extracts, it was still only an inhibition of 26.7%. In previous studies, it has been demonstrated that individuals allergic to both peanut and soybean have IgE antibodies that bind preferentially to larger proteins, while the antibodies of those reacting only to soybean bind strongly to proteins with lower molecular weight.23,24 In our study, we could not see a significant difference between the IgE binding pattern of pooled sera of both peanut and soybean positive subjects and soybean only positive subjects. Peanut only positive sera did not show significant cross-reactive IgE bands to soybean protein extracts either. Extensive cross-reactivity studies have been undertaken on the different legume species. And the majority of studies do not provide the clinical cross-reactions among the legume species.13,22 Nevertheless, as has been reported in a previous study, soy could be a cause of food anaphylaxis, and, as such, individuals with peanut allergies are at risk of a cross-reactive response.25 Individuals with peanut allergies should, therefore, be aware of this cross-reactivity, so that they can take the necessary measures if they have abnormal responses following ingestion of soybean.
In our study, three out of the eight sera that were positive for peanut but were negative for walnut in the immunoCAP test showed a significant IgE reactivity to walnut proteins in the immunoblot analysis. This contradictory result between the immunoCAP test and immunoblot analysis should be considered in the diagnosis of other food allergies, especially for individuals who are immunoCAP negative but still have allergic symptoms. However, the sera used for the experiments were collected based on the result of the immunoCAP test. Positivity by the immunoCAP test does not necessarily mean that the subject will show clinical symptoms following ingestion of the allergenic food. Therefore, future work needs to be undertaken with sera collected from symptomatic individuals.
Taken together, these results indicate that although the clinical significance of cross-reactivity of peanut with walnut and soybean has not been established, individuals with peanut allergies need to be cautious when consuming walnut and soybean.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflicts of interestNone declared.
This study was supported by a grant from the Korea Healthcare Technology R&D Project (A092076) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI11C1404 and HI14C0234). The authors thank Phadia Korea (Seoul, Korea) for the gift of the immunoCAP liquid reagents.