It is not quite well established how immune responses differ in term and preterm infants beyond the first year of life. This study aimed to evaluate aspects of the innate and adaptive immune responses in a group of preterm infants in comparison with their term peers.
MethodsIn this cross-sectional study peripheral blood mononuclear cells (PBMC) were isolated from preterm and term children at age three years. Innate immune response was evaluated by the analysis of TLR receptors expression on CD11c+HLADRhigh cells and inflammatory cytokine production after PBMC stimulation with Toll like receptors (TLR) ligands. Adaptive immune response was evaluated by T cells’ phenotyping and function after stimulation with polyclonal conventional T cell stimulus.
ConclusionWe have found that the patterns of innate and adaptive immune responses at 3 years of age were not affected by the fact of the children having being born preterm or at term.
Eleven percent of all infants are born premature (fewer than 37 weeks of gestation), and this represents almost 13 million children each year worldwide.1 In general terms, newborn babies have an increased susceptibility to infections due to the functional immaturity of their immune system.2 Preterm neonates present greater risk for morbidity and mortality, and this is in general associated with infection, since the immune system of preterm neonates is thought to be less developed at birth in comparison with term babies.3 However, this greater risk rapidly and significantly decreases during infancy,4 due to the progressing maturation process of the immune system. Protection against infectious pathogens is achieved through the coordinated actions of innate and adaptive immunity. There are few studies looking at leukocytes from preterm children and even less data available concerning the development of innate and adaptive immune systems comparing term and preterm babies in the first years of life.
Innate immune function is activated by sensors expressed by leukocytes. The best known innate sensors are pattern-recognition receptors (PRRs) of the Toll-like receptors (TLRs) family. Recent studies have demonstrated that the expression of TLRs in mononuclear cells of healthy infants over the first five years of life appears to be stable and to occur at adult-like levels.5–7 The cytokines released upon TLR stimulation have a potent regulatory effect, on both innate and adaptive immune cells. Dendritic cells (DC) are antigen presenting cells (APC), linking innate and adaptive immune response, controlling T cells differentiation. After contact with cognate MHC-peptide complexes on APCs, naïve T cells became effectors cells or memory T cells. It was previously demonstrated that the numbers of CD4 and CD8 T cells were lower in cord blood of preterm compared to term infants.8 Interestingly, the proportion of memory T cells (CD3+CD45RO+) was higher in peripheral blood of preterms compared to term babies at birth.9 However, reports on the development of adaptive immune response are scarce during this early maturation stage of the first years of life. The main objective of this study is to determine patterns of innate immune responses in term and preterm infants at age three years by characterising the TLR expression on dendritic cells and adaptive immune responses evaluating CD4 and CD8 T cells in peripheral blood mononuclear cells.
Materials and methodsEthics statementAll procedures involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all children's parents or guardians. This study was reviewed and approved by Research Ethics Committee of Pontifícia Universidade Católica do Rio Grande do Sul (CEP/PUCRS) under protocol number CEP10/04999.
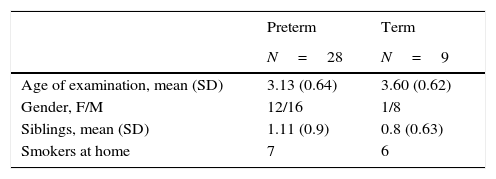
SubjectsThis is a cross-sectional study with data collected at a mean age of three years from children born preterm (mean gestational age 32.18 weeks) and full-term (mean gestational age>37 weeks). Patients were enrolled from 2011 to 2012. Exclusion criteria were viral respiratory infection or any other infection in the previous four weeks, chronic diseases (heart disease, neurological diseases, immunodeficiencies, allergic rhinitis and asthma) or the use of oral steroids or medications that could affect the results. Five millilitres of blood was collected from healthy preterm (n=28) and term children (n=9). Clinical data reported by parents or guardians and physical examination were collected by one of the investigators. Healthy term children were recruited among those undergoing an elective surgery, during the pre-operatory evaluation.
Mononuclear cell isolationPeripheral blood mononuclear cells (PBMCs) were purified from 5mL of whole blood using a Histopaque-1077 (Sigma–Aldrich, Saint Louis, MO, USA) gradient. Cells were frozen at −80°C until use. PBMCs were seeded at 2×105 cells/well in 96 well-culture plates with RPMI medium (Cultilab, Campinas, SP, Brazil), containing 2% of foetal calf serum (Cultilab, Campinas, SP, Brazil) and were stimulated with 2μg/mL of purified, no azide, low endotoxin, anti-CD3 (clone UCHT1) and anti-CD28 (clone CD28.2) antibodies (BD Bioscience, USA) or with TLR ligands: LPS from E. coli O111:B4 (10μg/mL) (Sigma–Aldrich, USA), PGN (peptidoglycan) from B. subtilis (4μg/mL) (Invivogen, San Diego, CA, USA), Poly I:C (Polyinosinic-polycytidylic acid) (1μg/mL) (Invivogen, San Diego, CA, USA) or left unstimulated for 24h. The culture supernatant was collected for cytokine analysis.
Flow cytometryTo analyse TLR expression and activation markers on DC, PBMCs were stained with the antibodies: anti-CD11c PE-Cy7 (clone B-ly6), anti-HLADR PE-Cy5 (clone TU36), anti-CD86 PerCP-Cy5.5 (2331 (FUN-1)), anti-TLR4 Biotin (clone HTA 125) followed by FITC Streptavidin, and anti-CD282 (TLR2) Alexa Fluor® 647 (clone 11G7). To analyse memory T cells, PBMCs were stained with: anti-CD3 APC (clone UCHT1), anti-CD4 PECy7 (clone SK3), anti-CD8 FITC (clone RPA-T8), anti-CD45RO PerCP Cy 5.5 (clone UCHL1), and anti-CD27 APC-H7 (clone M-T271) antibodies. To evaluate the expression of intracellular TLR and granzyme B, cells were permeabilised using Cytofix/Cytoperm kit and stained with anti-TLR9 PE (clone eB72 1665) and anti-Granzyme B Alexa Fluor®647 (clone GB11). All antibodies were from BD Bioscience (USA). Data were accessed by flow cytometry using FACS Canto II (BD Bioscience) and analysed using Flow Jo software (Tree Star). Cytokine analysis from cell culture supernatant was performed using CBA (Cytometric Bead Array) Inflammation kit (BD Bioscience, USA) followed by analysis on FCAPArray 3.0 (Soft Flow).
Statistical analysisData were tested for normal distribution with the Kolmogorov–Smirnov test using SPSS software 17.0 (IBM, New York, USA). Kruskal–Wallis test followed by Dunn post-test and Mann–Whitney U test were applied to non-parametric data using GraphPad Prism software 5.0 (San Diego, CA, USA). Values demonstrated in tables and graphs are median and interquartile range. The level of significance was set at p<0.05.
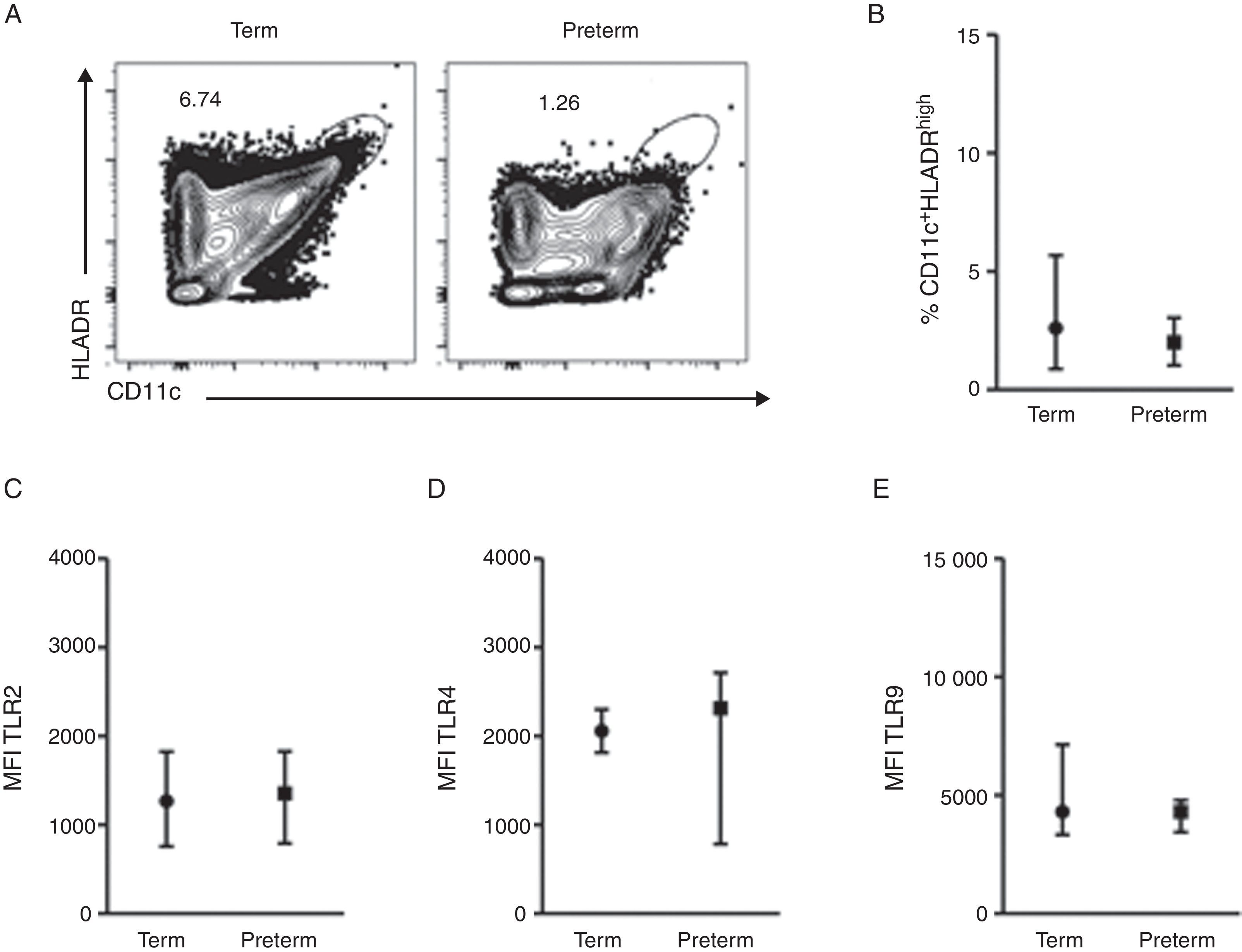
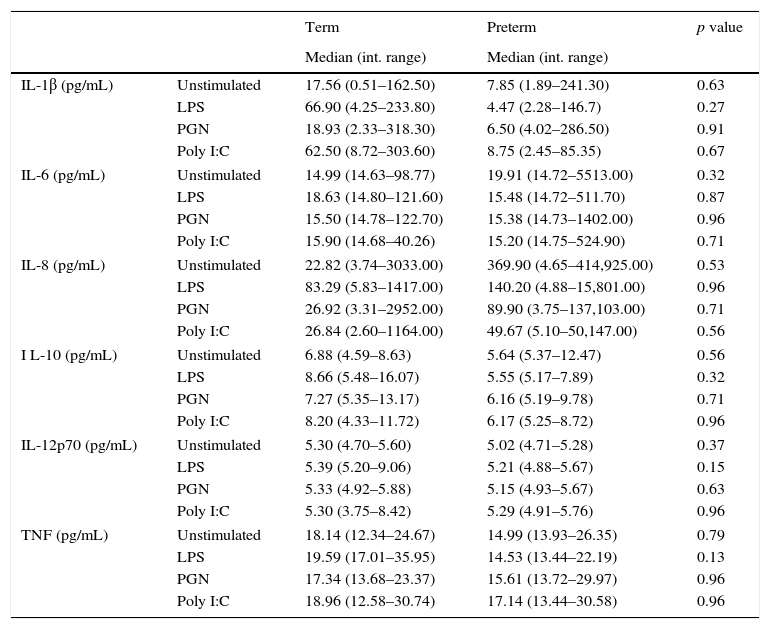
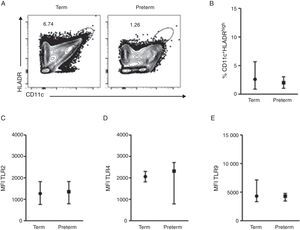
ResultsA total of 37 subjects were enrolled in this study. Children born preterm and term had a mean age of 3.13 and 3.6 years, respectively. Demographic data are shown on Table 1. CD11c+HLADRhigh dendritic cells (DC) are a heterogeneous population of antigen-presenting cells that are critical to prime T cells and modulate the immune response, especially by the expression of co-stimulatory molecules, TLRs and cytokine production.10 In order to evaluate TLR expression on CD11c+HLADRhigh cells, PBMCs were purified from whole blood of children born preterm or full-term aged three years, stained with antibodies and analysed by flow cytometry. Fig. 1A shows the gate strategy of CD11c and HLADR positive cells analysis. There was no significant difference (p=0.72) (Fig. 1B) in the frequency of CD11c+HLADRhigh cells between children born preterm (median 2.1%) or term (median 2.6%). They also presented a similar MFI (Mean Fluorescence Intensity) for TLR2, TLR4 and TLR9 receptors on CD11c+HLADRhigh cells (Fig. 1C, D and E). These results suggest that at three years of age, CD11c+HLADRhigh DCs from preterm children express equivalent levels of TLR receptors compared to those of term children. Cytokine production after PBMC stimulation with TLR ligands (LPS, PGN and Poly I:C) was also evaluated in those groups. No significant differences in IL-1β, IL-6, IL-8, IL-10, IL-12p70 and TNF secretion were found between the two groups (Table 2). Those data indicated that children born either preterm or at term present a similar pattern of innate immune response at age three years.
TLR4, TLR2 and TLR9 expression on peripheral blood mononuclear cells from preterm and full-term children at three years of age. PBMC were isolated and stained with anti-CD11c, anti-HLADR, anti-TLR2, anti-TLR4 e anti-TRL9 antibodies. (A) Dot plots showing the frequency of CD11c+HLADRhigh cells from preterm and term children. (B) Frequency of CD11c+HLADRhigh cells (p=0.72). (C) MFI (Mean Fluorescence Intensity) of TLR2 in CD11c+HLADRhigh cells (p=0.81). (D) MFI of TLR4 in CD11c+HLADRhigh cells (p=0.92). (E) MFI of TLR9 in CD11c+HLADRhigh cells (p=0.62). Data are expressed as median and interquartile range.
Supernatants cytokine analysis of unstimulated and stimulated peripheral blood mononuclear cells (PBMC) from term and preterm children.
| Term | Preterm | p value | ||
|---|---|---|---|---|
| Median (int. range) | Median (int. range) | |||
| IL-1β (pg/mL) | Unstimulated | 17.56 (0.51–162.50) | 7.85 (1.89–241.30) | 0.63 |
| LPS | 66.90 (4.25–233.80) | 4.47 (2.28–146.7) | 0.27 | |
| PGN | 18.93 (2.33–318.30) | 6.50 (4.02–286.50) | 0.91 | |
| Poly I:C | 62.50 (8.72–303.60) | 8.75 (2.45–85.35) | 0.67 | |
| IL-6 (pg/mL) | Unstimulated | 14.99 (14.63–98.77) | 19.91 (14.72–5513.00) | 0.32 |
| LPS | 18.63 (14.80–121.60) | 15.48 (14.72–511.70) | 0.87 | |
| PGN | 15.50 (14.78–122.70) | 15.38 (14.73–1402.00) | 0.96 | |
| Poly I:C | 15.90 (14.68–40.26) | 15.20 (14.75–524.90) | 0.71 | |
| IL-8 (pg/mL) | Unstimulated | 22.82 (3.74–3033.00) | 369.90 (4.65–414,925.00) | 0.53 |
| LPS | 83.29 (5.83–1417.00) | 140.20 (4.88–15,801.00) | 0.96 | |
| PGN | 26.92 (3.31–2952.00) | 89.90 (3.75–137,103.00) | 0.71 | |
| Poly I:C | 26.84 (2.60–1164.00) | 49.67 (5.10–50,147.00) | 0.56 | |
| I L-10 (pg/mL) | Unstimulated | 6.88 (4.59–8.63) | 5.64 (5.37–12.47) | 0.56 |
| LPS | 8.66 (5.48–16.07) | 5.55 (5.17–7.89) | 0.32 | |
| PGN | 7.27 (5.35–13.17) | 6.16 (5.19–9.78) | 0.71 | |
| Poly I:C | 8.20 (4.33–11.72) | 6.17 (5.25–8.72) | 0.96 | |
| IL-12p70 (pg/mL) | Unstimulated | 5.30 (4.70–5.60) | 5.02 (4.71–5.28) | 0.37 |
| LPS | 5.39 (5.20–9.06) | 5.21 (4.88–5.67) | 0.15 | |
| PGN | 5.33 (4.92–5.88) | 5.15 (4.93–5.67) | 0.63 | |
| Poly I:C | 5.30 (3.75–8.42) | 5.29 (4.91–5.76) | 0.96 | |
| TNF (pg/mL) | Unstimulated | 18.14 (12.34–24.67) | 14.99 (13.93–26.35) | 0.79 |
| LPS | 19.59 (17.01–35.95) | 14.53 (13.44–22.19) | 0.13 | |
| PGN | 17.34 (13.68–23.37) | 15.61 (13.72–29.97) | 0.96 | |
| Poly I:C | 18.96 (12.58–30.74) | 17.14 (13.44–30.58) | 0.96 | |
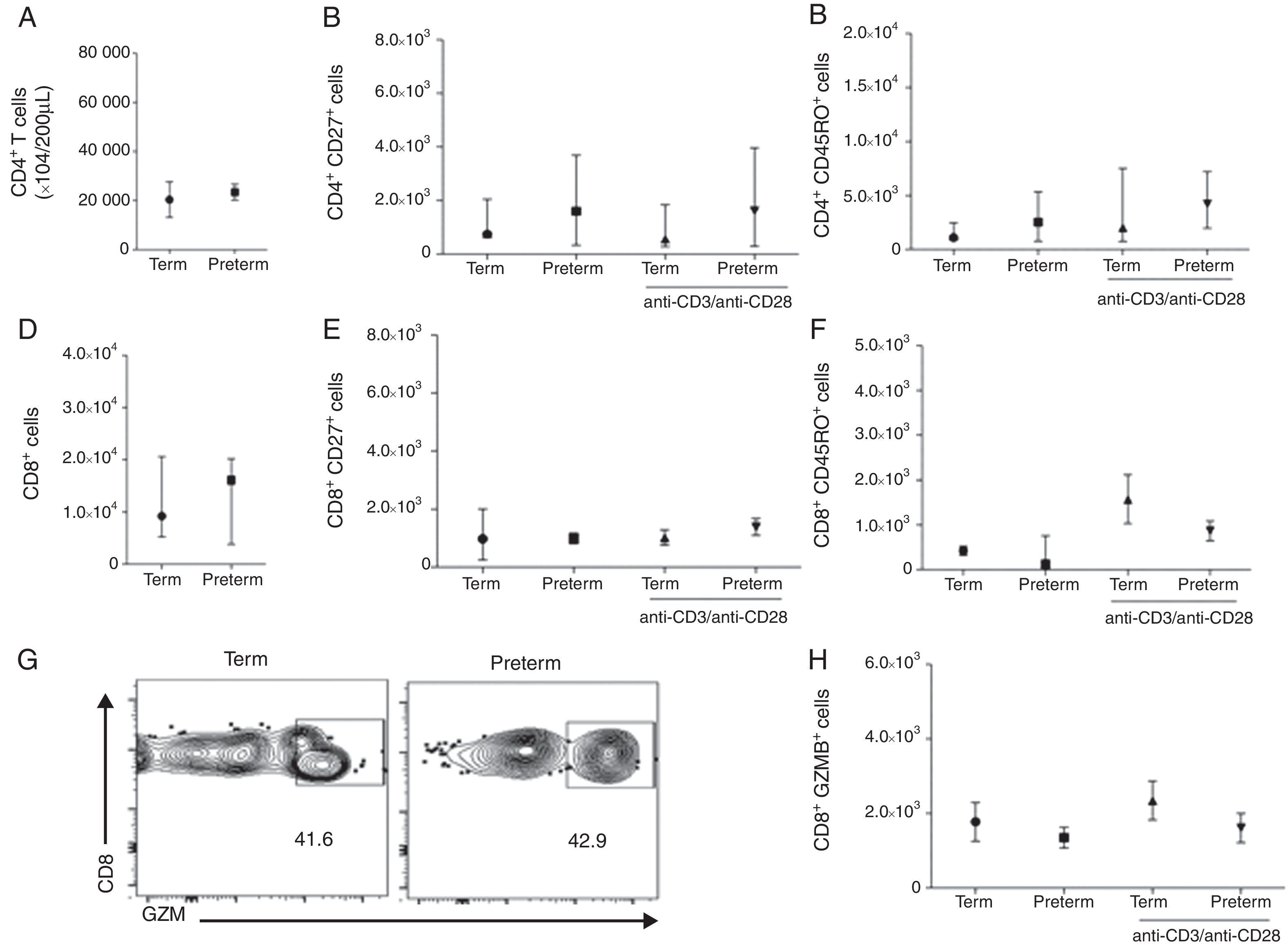
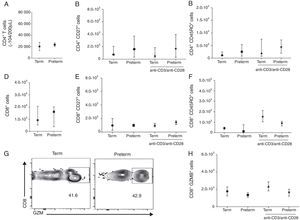
We than asked whether the adaptive immune response between these two groups of children would also be comparable. To evaluate this, we analysed CD4 and CD8 T cell phenotypes by stimulating PBMCs with a T cell polyclonal stimulus. Preterms present similar number of CD4 T cells (median 2.1×104 cells/200μL) when compared to term (median 1.2×104 cells/200μL) (p=0.58) (Fig. 2A). We further analysed the memory phenotype of CD4 T cells by stimulating cells with anti-CD3/anti-CD28 antibodies for 24h. Preterm children presented similar percentages of CD4+CD27+ and CD4+CD45RO+ T cells in peripheral blood when compared with term children. Furthermore, anti-CD3/anti-CD28 stimulation did not alter the numbers of CD4+CD27+ and CD4+CD45RO+ T cells in both preterm and term groups (Fig. 2B and C). These data suggested that memory CD4 T cells responses in preterm were preserved in preterm children and comparable to full-term ones. Moreover, the number of CD8 T cells was similar between the two groups (Fig. 2 D). In addition, we have analysed the memory phenotype of CD8 T cells and their function through granzyme B production. Our data showed a similar number of CD8+CD27+ and CD8+CD45RO+ T cells among preterm and term children. Again, anti-CD3/anti-CD28 stimulation did not change the numbers of CD8+CD27+ and CD8+CD45RO+ T cells in both groups of children (Fig. 2E and F). Analysing the number of CD8+ T cells producing granzyme B, we found that at age three years, children born either at term or preterm had a similar number of these cells (Fig. 2G and H), suggesting that the immunological role of these cells in controlling infections is not impaired among those born prematurely.
T cell analysis of preterm and full-term children at three years of age. PBMC were isolated and T cells were stimulated with anti-CD3 and anti-CD28 antibodies for 24h and stained with anti-CD3, anti-CD8, anti-CD4, anti-CD27, anti-CD45RO and anti-granzyme B antibodies. (A) Number of CD4+ T cells (p=0.58). (B) Number of CD4+CD27+ T cells (p=0.52). (C) Number of CD4+CD45RO+ T cells (p=0.25). (D) Number of CD8+ T cells (p=0.95). (E) Number of CD8+CD27+ T cells (p=0.91). (F) Number of CD8+CD45RO+ T cells (p=0.14). (G) Dot plots of the frequency of CD8+GZMB+ cells. (H) Number of CD8+GZMB+ T cells (p=0.26). Data are expressed as median and interquartile range.
This study demonstrates that children born preterm and at term present similar patterns of innate and adaptive immune responses years after birth. The development of the human immune system begins early in the foetal period and it seems not to be completely functional at the time of birth. Post-natal maturation proceeds for months to years, even into adulthood. Preterm infants present high vulnerability to infections,11–14 possibly due to differences in the number and function of immune cells. One of these differences in preterm neonates is associated with innate immune functions, including the PRR function analysed in cord or peripheral blood.2,15,16 CD11c+HLADRhigh DC are critical to prime T cells efficiently and modulate the immune response, especially by the expression of co-stimulatory molecules, TLR receptors and cytokine production. We could not find any significant differences for the frequencies of CD11c+HLADRhigh DC between preterm and full-term children. A previous study has shown that DC from term children gradually increase the expression of HLADR on their surfaces within the first six months of life, then reaching adult-like levels.17 In our study, DC from preterms behave as DC from term children, acquiring the expression of HLADR and maintaining equivalent DC numbers later in life. These data indicate that the development of the immune system after birth is different between term and preterm but they become indistinguishable at age three years.
At age three, children born at term or preterm present similar expression of TLR4, TLR2 and TLR9 receptors on CD11c+HLADRhigh cells. A recent study has shown similar expression of TLR-2 and TLR-4 receptors on monocytes among preterm and term new-borns.2 Another study has demonstrated that the monocyte expression of TLR-2 and TLR-4 in preterm new-borns increased rapidly within the first two weeks after birth, reaching the expression levels of term new-borns. These increases continued in the following 4–6 weeks, when they reached a plateau.18 Altogether, these data suggest that TLR expression on monocytes from pre-terms rapidly reaches that of those born at term and these expression levels are maintained at least until age three years.
Recent studies have shown that the levels of IL-6, IL-8, IL-12 and TNF were lower in the sera of preterm infants (born between 30–32 weeks of gestation) compared to infants born after 36 weeks.13,19 These authors assumed that the lower levels of cytokines found in preterm infants are related to a lower stimulation or activation of monocytes and dendritic cells. We, however, found an equivalent level of stimulation of PBMCs, since there was no difference in cytokine secretion among cells from preterm and term children. Our results suggest that children born preterm have similar frequencies of DC and that these cells are able to respond to an inflammatory stimulus, when called into action.
CD8 T cytotoxic cell and CD4 T helper cells are important to control infections and memory T cells can provide life-long protection against pathogens.20 Given that, we analysed CD4 and CD8 T cell in peripheral blood of our study population. Although those born preterm had lower counts of T helper cells than term infants when tested 7–8 weeks of age comparing with term infants,21 at age three years these differences were no longer significant. However, analysis of T cells of very preterm children (<30 weeks) at 8.8 years old, has previously showed low CD4/CD8 ratios due to the low number of CD4 T cells.21 These data are different from ours but the populations are not comparable either; our sample did not include very preterm children. Intriguingly, the CD45RO memory marker on T cells was higher in cord blood from preterm children compared to term.9 In contrast, another study was not able to detect any difference related to naïve and memory T helper cells in the cord blood of preterm children when compared with term neonates.2
At age three years, our results are quite similar to those described above. We did not find significant differences between children born either at term or preterm in relation to CD4+CD45RO+, CD4+CD27+, CD8+CD45RO+ and CD8+CD27+ levels on peripheral blood. In addition, we have extended our analysis in order to evaluate CD8 T cells function by measuring granzyme B production and have not detected any significant differences of CD8 T cell production of granzyme B, which suggests that their function is well preserved.
The major limitation of our study was the small number of subjects included. This was difficult to overcome because of the difficulty of recruiting healthy preterm and term children at this age in order only to collect blood samples. However, given that this type of data is scarce in the literature, we believe that our results are valuable and will help raising further questions on the development of the immune response in preterm-born children.
In conclusion, our data show that deficiencies in the patterns of innate and adaptive immune responses at birth do not necessarily mean that it does not go through an evolutionary and maturational process during the first years after birth. We showed that preterm children at three years of age present a functional immune system to defend them against infections, with similar frequency of dendritic and T cells, as well as similar expression of pattern recognition receptors and cytokine production, when compared to term children at this same age.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestAll authors have no conflict of interest to declare.
Author's contributionS. P. M., acquisition and analysis of data. P. M. P., interpretation of data, drafting the work and revising it critically. A. P. D. S., acquisition and analysis of data, interpretation of data, drafting the work and revising it critically. B. N. P., acquisition and analysis of data, interpretation of data, drafting the work and revising it critically. J. E. V., acquisition and analysis of data, interpretation of data, drafting the work and revising it critically. I. P. E., acquisition and analysis of data, interpretation of data, drafting the work and revising it critically. J. P. H.-F., acquisition and analysis of data. G. S., acquisition and analysis of data. T. S. B., acquisition and analysis of data. T. D. G., acquisition and analysis of data. F. D. M., acquisition and analysis of data. M. H. J., interpretation of data, drafting the work and revising it critically. C. B., interpretation of data, drafting the work and revising it critically. R. T. S., interpretation of data, drafting the work and revising it critically.
This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Grant Number 02775/09-3, and Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS), Grant Number 10/1313-9. Souza, A. P. D., Porto, B. N., Vargas, J. E., Ewald, I. P. received post-doctoral fellowships from CAPES-PNPD program. The authors thank Rodrigo Godinho de Souza for the excellent technical assistance.