Alteration in the proliferation capacity of leukocytes and in the level of some cytokines, such as TNF-α, IL-4 and IL-8 have been suggested to associate with Diabetes mellitus in alloxan-induced diabetic rats given the potential immunomodulatory effects of black seeds and garlic.
Aim of the workThe aim of this study was to test the effects of these agents on the immune cells in alloxan-induced diabetic rats.
MethodsTo this end, Diabetes was induced in albino rats by a single intraperitoneal injection of alloxan monohydrate (120mg/kg of body weight). Diabetic rats were then fed normal diet or diet with black seeds or garlic for 28 days.
ResultsThe results showed significant increase in the numbers of monocytes and granulocytes, but with significant decreases in lymphocyte proliferation and the TNF-α, interleukin (IL)-4 and IL-8 levels in the diabetic group. Treatment of diabetic rats with black seeds or garlic induced significant amelioration in the numbers of monocytes and granulocytes, with significant increase in lymphocytes numbers and the TNF-α, IL-4 and IL-8 levels.
ConclusionsThese results indicate the potential beneficial effects of black seeds and garlic as adjuvant treatment during treatment of Diabetes.
Medicinal plants have been used to cure human illness since ancient times. Certain types of these plants are believed to promote positive health and maintain organism resistance against infection by re-establishing body equilibrium and conditioning the body tissues. Among these plants, Nigella sativa, an annual herbaceous plant that belongs to family Ranunculaceae, has been used for thousands of years in traditional medicine. Its seeds are claimed to have anticancer, antileukaemic and antimicrobial effects.1 The seeds have been found by some authors to possess an immunopotential activity.2 Garlic (Allium sativum) also has traditional dietary and medicinal applications as an antimicrobial agent.3 Garlic is a common food spice widely distributed and used in all parts of the world as a spice and herbal medicine for the prevention and treatment of a variety of diseases, ranging from infections to heart diseases.4 Garlic is thought to have various pharmacological properties and medical applications. It is mainly consumed as a condiment in various prepared foods.5
Diabetes mellitus is a syndrome of impaired carbohydrate, fat, and protein metabolism caused by limited insulin secretion or lower tissue-sensitivity to insulin. This metabolic disorder is frequently diagnosed by hyperglycaemia, lipid abnormalities and vascular complications.6 In animals with experimental Diabetes, there is suppression of cellular immunity and reduced mitogenic response to several antigens.7 It has long been recognised that the inflammatory response in diabetic patients is impaired.8–13 Alloxan has been used to induce Diabetes into experimental animals. It acts through selective uptake by low affinity GLUT2 glucose transporter into the beta-cell leading to the destruction of the transporter protein by oxygen free radicals.14 Significant reduction in the body weight gain and hyperglycaemia are present in rats after alloxan injection.15
Alterations in lymphocytes are a common finding in both type I and type II Diabetes.16 Abnormalities of leukocyte function have been shown to occur during inflammation in alloxan-induced diabetic rats,8 including reduced number of leukocytes in inflammatory lesions17 and reduced production and transcription of pro-inflammatory (TNF-α) cytokines.8,9 Lymphocyte dysfunction might be the main cause of higher incidence of infections in diabetics, since an increased number of apoptotic lymphocytes were found in alloxan-induced diabetic animals and diabetic patients.18 Peripheral blood mononuclear cells from diabetic patients are reduced in their ability to produce cytokines19 and the proliferate responses of CD4+ T-cells to primary protein antigens are significantly reduced.20 Some authors suggest that metabolic disturbances and therapeutic efforts to restore glucose metabolism may underlie part of the observed changes in lymphocyte subsets in either diabetic type.21 Elevated serum levels of IL-8, a potent chemoattractant for neutrophils and T-lymphocytes, were found in type 1 and 2 diabetic subjects, suggesting that this cytokine might contribute to the development of diabetic macroangiopathy.22
The aim of this work was to evaluate the beneficial effects of black seeds and garlic on the altered leukocyte number and function as well as the levels of TNF-α, IL-4 and IL-8 in alloxan-induced diabetic rats with non-diabetics.
Materials and methodsCrude extractBlack seeds and garlic cloves purchased from the local market in El-Minia, Egypt were used. The animals were allowed free access to food 50g of seeds and cloves daily during the course of the treatment, after fasting for about 12h.
AnimalsMale albino rats (Rattus norvegicus), weighing 120–150g and of average age four months, were obtained from the Biological Supply Center, Theodore Bilharz Research Institute, TBRI, Cairo, Egypt and housed under specific pathogen-free conditions and maintained on a 12-h light-dark cycle, with food and water ad libitum. Animals were divided into four groups (10 animals each). The first group (control) was untreated. The second, third and fourth groups were artificially Diabetes induced by intraperitoneal injection with alloxan (Sigma Chemical Co., USA) 120mg/kg of body weight, freshly dissolved in 5mmol sterile normal saline.23 The third group was intraperitoneal injected with alloxan once during the first week and allowed free access to food 50g of black seeds daily during the course of the treatment, after fasting for about 12h. The fourth group was treated similarly to the third group but with garlic instead of black seeds. Rats with blood glucose levels ≥250mg/dl were considered to be diabetic. Plasma glucose levels in the control animals remained normal throughout the study.
Quantification of serum cytokinesThe concentrations of TNF-α, IL-4 and IL-8 in collected serum samples were determined by enzyme-linked immunosorbent assay (ELISA) using commercially available kits according to the manufacturer's instructions (R&D Systems Inc., Minneapolis, MN, USA). The sensitivity of the assays was 15pg/ml.
Leukocyte differential countFreshly collected blood samples of about 20μl were spread on clean slides as a thin film using another smooth-edged glass slide. Each blood smear was left to air dry before being fixed with methanol for 2–3min and then labelled by the number of the animal. Blood smears were stained with 10% Giemsa's stain (Aldrich) in buffered distilled water containing 0.021M Na2HPO4/0.015M KH2PO4. pH 7–7.2 for 30min and kept away from sunlight. After that, the stain was removed by gentle washing with distilled water and the slides were air-dried at room temperature.24 Using light microscopy at 400× magnification, different types of blood leukocytes were recorded. At least double smears for each blood sample were counted.
Statistical analysisData were analysed using SPSS program version 13.0. Statistical analysis of the obtained data was performed using one way analysis of variance (ANOVA) test followed by least square differences (LSD) analysis for comparison between means. Results were expressed as mean±standard error (SE). Values of P<0.05 were considered statistically significant, while values of P>0.05 were considered statistically non-significant.
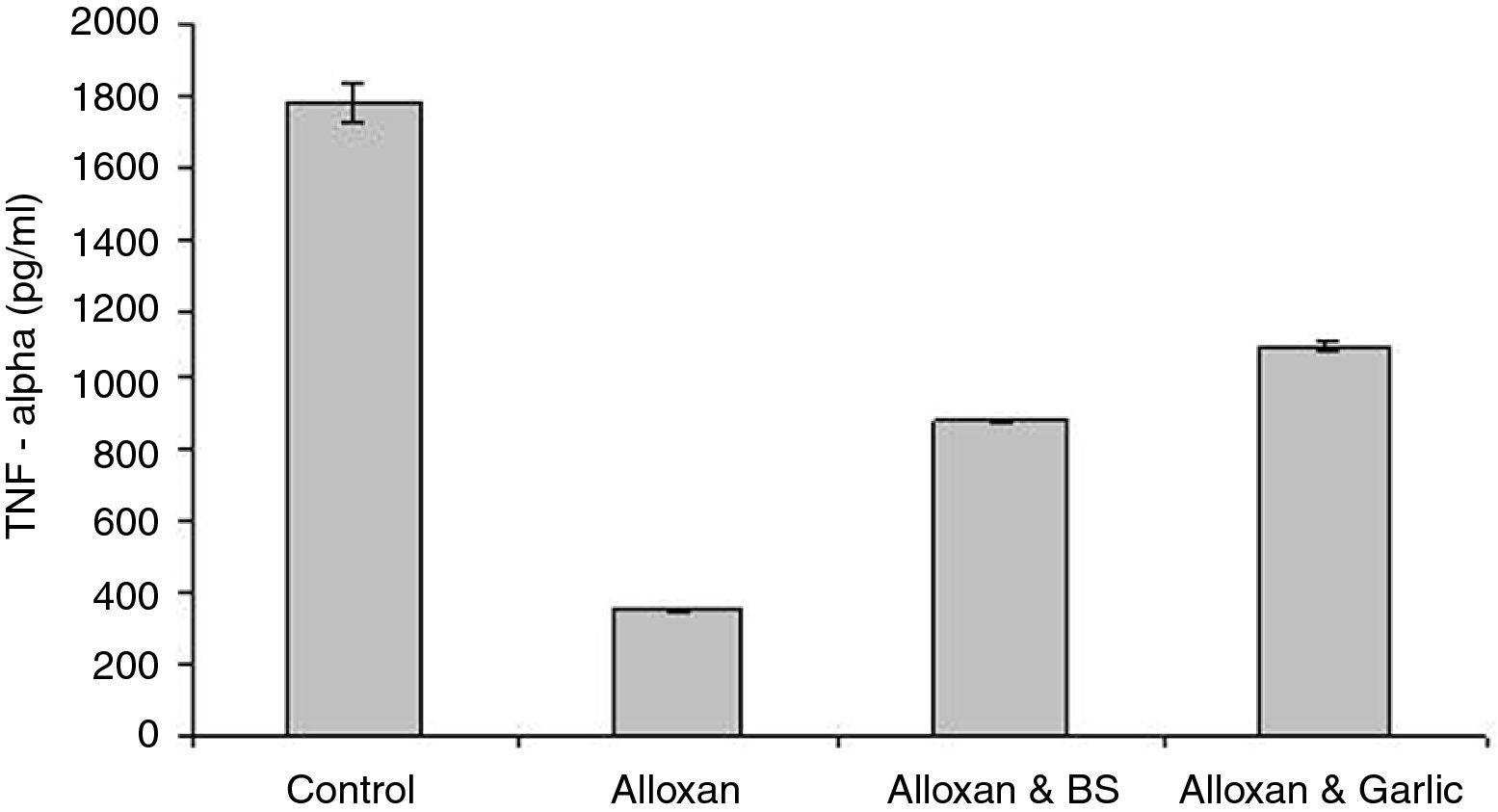
ResultsQuantification of TNF-α in seraThe TNF-α ELISA values demonstrated that the control group (1780.39±52.621pg/ml) is higher than the diabetic group (352.59±4.204pg/ml) which is lower than the diabetic group treated with either black seeds or garlic, counting 882.69±3.468pg/ml and 1100.28±10.858pg/ml, respectively (Fig. 1). This study showed a significant difference in TNF-α level between all groups (P<0.05).
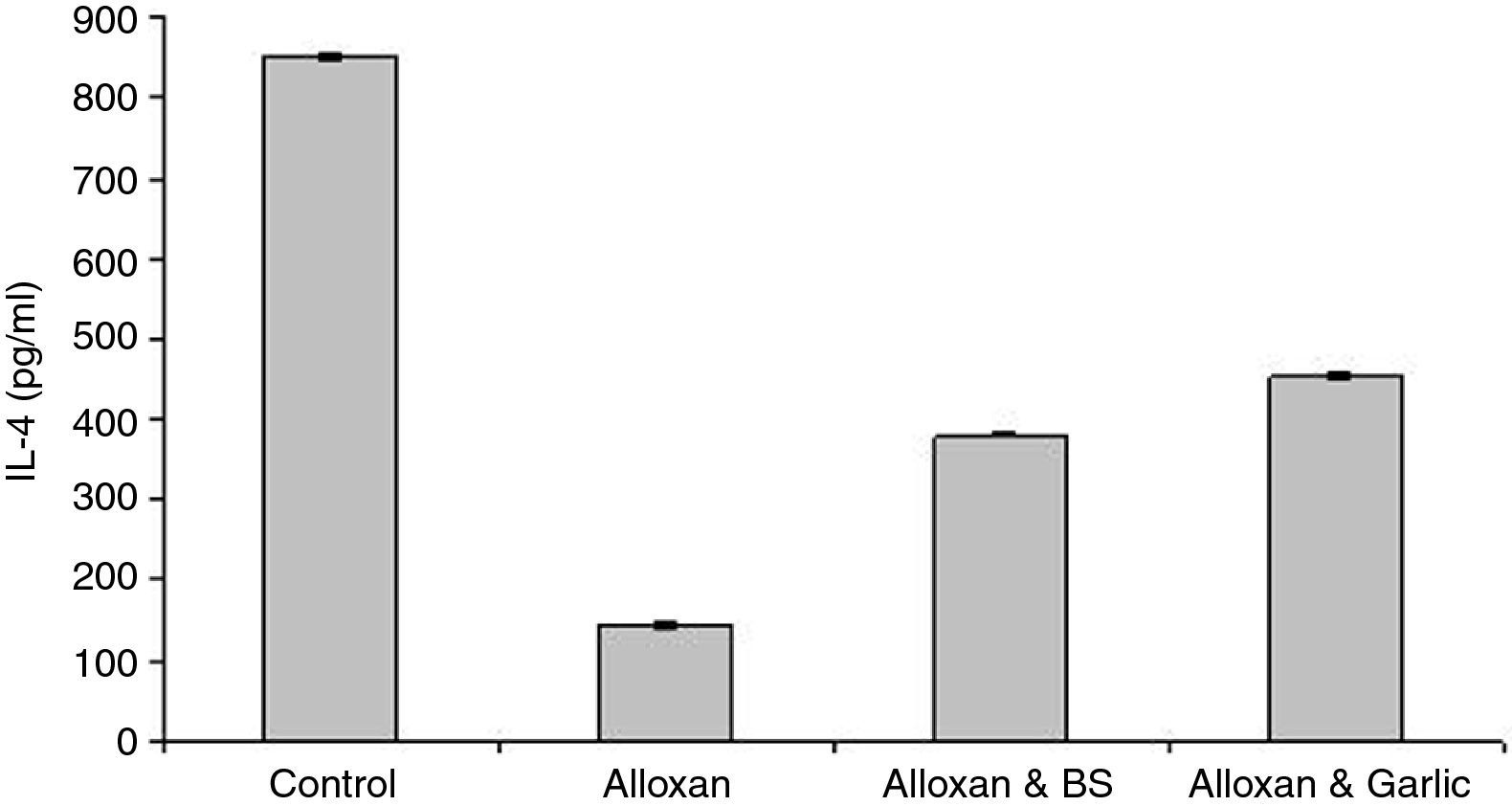
Quantification of serum IL-4The results of ELISA in Fig. 2 showed that IL-4 recorded 850.83±3.581pg/ml in the control group. The diabetic group showed a lower level of IL-4 (144.47±5.319pg/ml) than the diabetic groups treated with either black seeds or garlic (379.20±3.060pg/ml and 452.16±6.041pg/ml, respectively). Statistical analysis showed a significant difference in IL-4 between the diabetic group and either the control group or the diabetic group treated with black seeds and garlic (P<0.05).
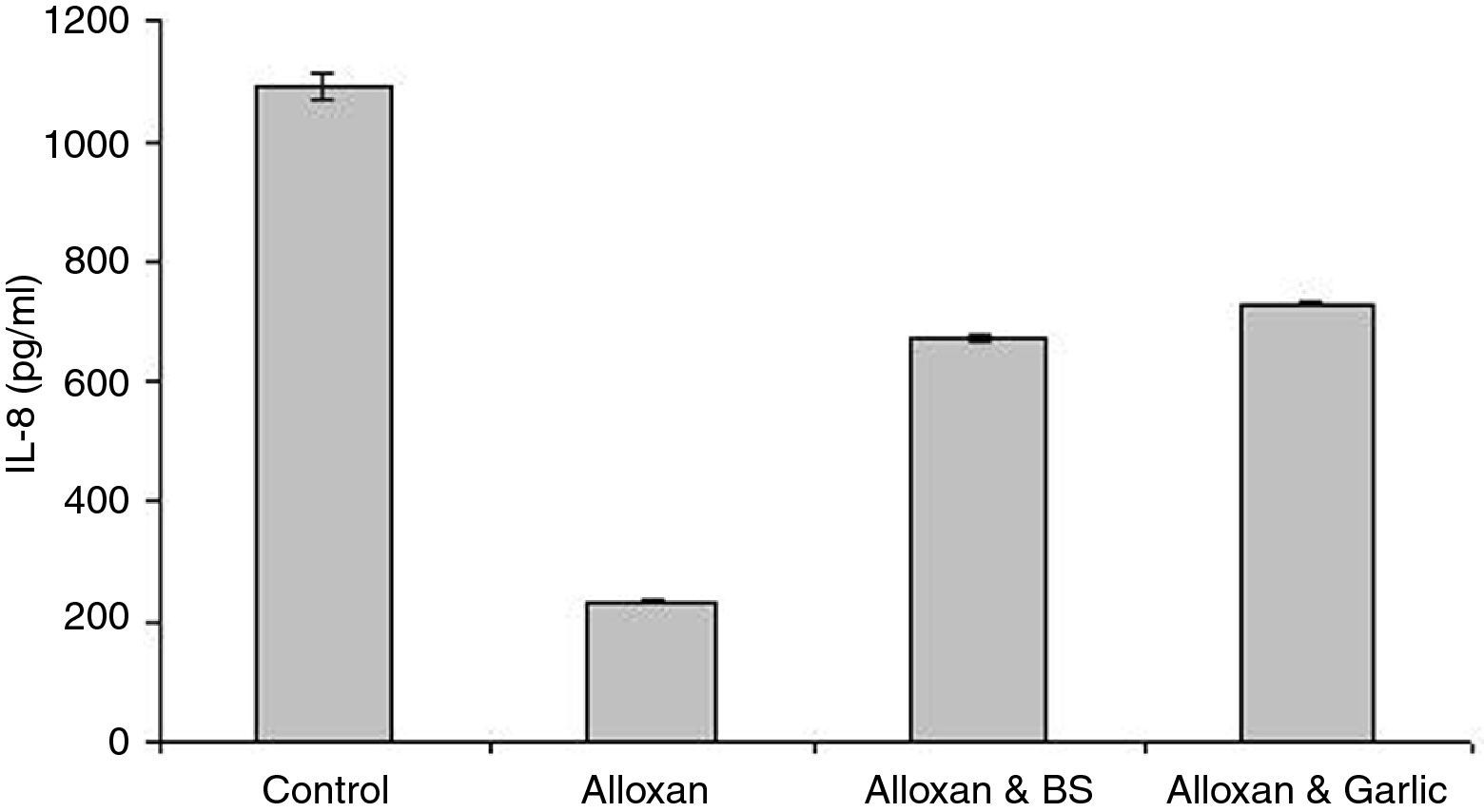
Quantification of serum IL-8In parallel to TNF-α and IL-4, ELISA tests of IL-8 showed that the diabetic group treated with black seeds (673.59±3.243pg/ml) or with garlic (731.02±3.117pg/ml) is higher than the diabetic group (232.10±4.304pg/ml), but lower than the control group (1090.21±23.077pg/ml). These differences can be seen in Fig. 3. Significant differences in IL-8 levels were monitored between the diabetic group and either the control group or the diabetic group treated with both black seeds and garlic (P<0.05).
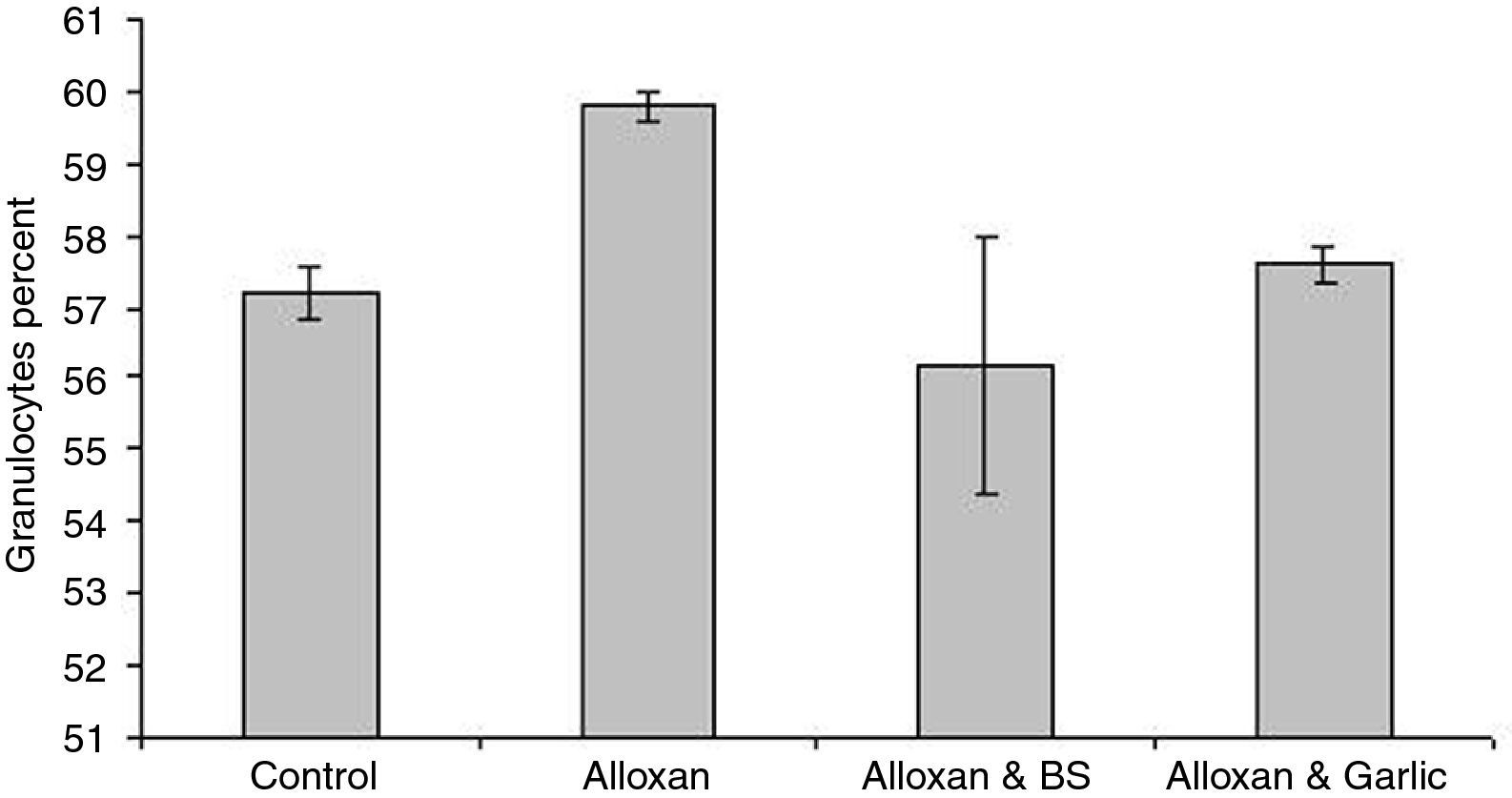
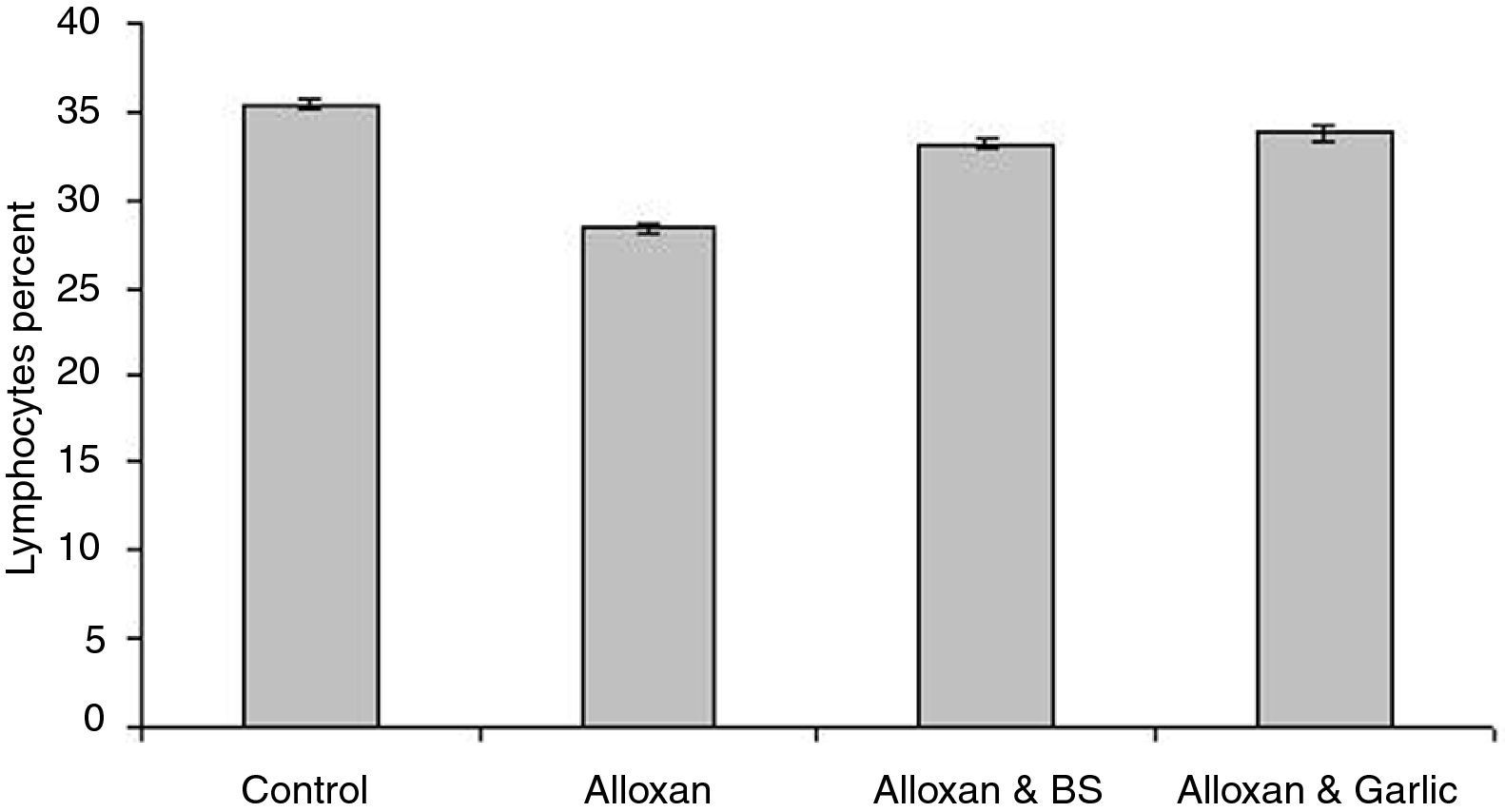
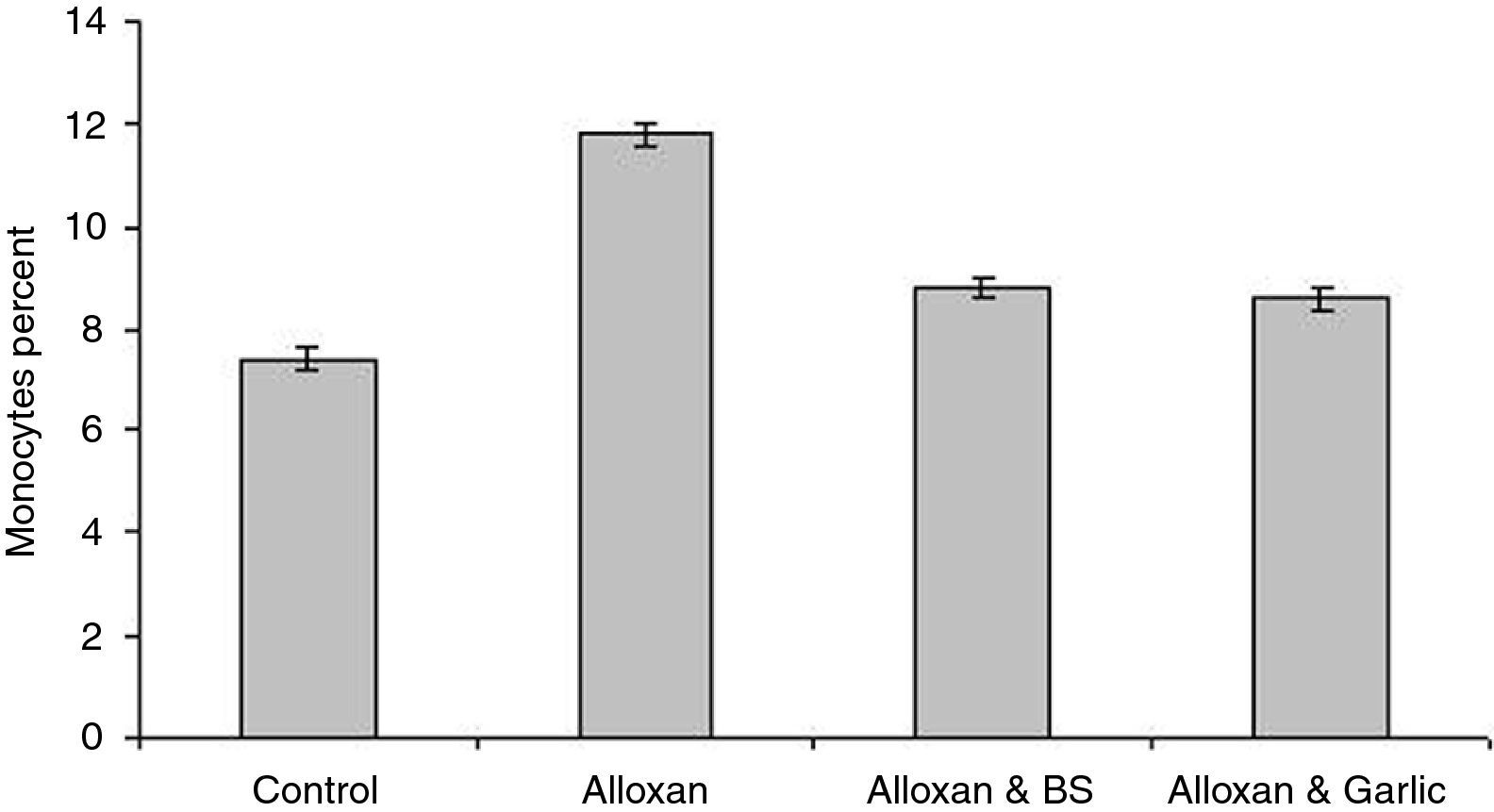
Leukocyte proliferationFig. 4 shows that granulocytes percentage in the diabetic group (59.80±0.200) is higher than both the control group (57.20±0.374) and the diabetic group treated with black seeds (56.20±1.800) and garlic (57.60±0.245). On the other hand, lymphocytes percent in the diabetic group (28.40±0.245) is slightly lower than both the control group (35.40±0.245) and the diabetic group treated with black seeds and garlic recording 33.20±0.200 and 33.80±0.490, respectively (Fig. 5). In parallel to granulocytes, Fig. 6 shows that the monocytes percentage in the diabetic group (11.80±0.200) is higher than both the control group (7.40±0.245) and the diabetic group treated with black seeds and garlic (8.80±0.200 and 8.60±0.245, respectively).
Statistical analysis showed that there is a significant difference in leukocyte count between the diabetic groups and either the control group or the diabetic group treated with both black seeds and garlic (P<0.05).
DiscussionAlloxan has been known to be diabetogenic and induces DNA strand breaks in isolated rat pancreatic islets in vitro to cause Diabetes mellitus.25 This is in accordance with our data, where alloxan injection showed statistically significant increase in glucose concentration versus control groups (P<0.05). This rise in the glucose was significantly reduced after black seeds and garlic treatment compared to the glucose level in alloxan-treated groups. The mechanism of action of black seeds or garlic to decrease the glucose concentration in Diabetes mellitus is still unknown. Oral supplementation of black seeds after alloxan treatment resulted in lower plasma glucose levels and higher serum insulin values as compared with alloxan alone.26 So, the decrease of glucose level in the black seeds and garlic treated groups revealed that these medicinal plants might be involved in reducing or inhibiting alloxan DNA strand breaks, increasing insulin secretion and consequently decreasing the glucose level.
Reinhold et al. showed that elevated glucose concentration significantly affected cytokine production.27 In accordance, the results presented here showed changes in the level of the pro-inflammatory cytokine, TNF-α, the anti-inflammatory cytokine, IL-4, and the chemo-attractant cytokine, IL-8, between the diabetic group and the other groups. The changes in the TNF-α level could be due to the changes in insulin secretion, where insulin modulates the development of the inflammatory reaction to allergen challenge by its ability to modulate the production/release of TNF-α.10 In contrast to our data, a significant increase in IL-8 concentration in alloxan groups versus control groups (P value<0.05) and a significant decrease after treatment compared with alloxan groups were detected.28 In addition, circulating levels of IL-8 have been reported to be increased in patients with type 1 and type 2 Diabetes linking these chemokines with insulin resistance.22
Leukocytes proliferation results suggested that alloxan-diabetic rates had an increase in granulocytes and monocytes, and a decrease in lymphocytes proliferations. On the other hand, the diabetic rats treated with black seeds and garlic showed modulations in these changes. These are supported by a number of previous observations: (1) high glucose concentrations modified lymphocyte proliferate capacity29; (2) Reinhold et al. showed that elevated glucose concentration significantly inhibits peripheral mononuclear cell proliferation by inhibiting the endogenous production of IL-2, IL-6 and IL-1027; (3) hyperglycaemia in alloxan diabetic mice non-specifically impedes, but does not completely inhibit, the proliferation and growth of lymphocyte populations.30
In contrast to our results, Martins et al. showed a significant reduction in the number of neutrophils and mononuclear leukocytes.15 They also mentioned that a complete recovery of the impaired responses was observed under the influence of insulin.
In conclusion, the data presented here showed that both black seeds and garlic could be involved in modulation of some immunological disorders induced by alloxan, when they were injected in combination.
I thank Dr. Mohamed Abuel-Maged (Zoology Department, Faculty of Science, Minia University, El-Minia, Egypt) for revising the manuscript.