The primary role of infections in chronic urticaria (CU) is controversial. We hypothesised that streptococcal tonsillitis (ST) could be a primary cause of CU or acute recurrent urticaria (ARU).
MethodsRetrospective study of 14 outpatients observed between January 2000 and December 2009, with CU/ARU and clinical and/or laboratorial suspicion of an aetiopathogenic link with ST. Clinical history, objective examination and laboratorial study were looked for. Three groups were defined: spontaneous resolution of urticaria, resolution after tonsillectomy, and still symptomatic.
ResultsIn these patients, a causal relationship between ST and urticaria is supported by: markers of streptococcal infection, the perception of a clinical relationship between tonsillitis and urticaria, the decrease of urticaria severity with early antibiotherapy to tonsillitis and urticaria resolution after tonsillectomy.
ConclusionsOur study encourages the investigation of tonsillitis in these otherwise idiopathic patients, especially until young adulthood and even in the absence of any symptoms.
Chronic urticaria (CU) can be a sign/symptom of a subjacent organic disease, systemic or limited to the skin.
The causal relationship between infections and acute urticaria is consensual.1 Infections are also well recognised as an exacerbating factor of CU.2 Besides infections and CU relationship had been published as early as in 1926,3 a primary role of infections in CU still controversial.
CU has been reported to be associated with a wide number of infectious agents: bacteria (Streptococcus and Mycoplasma species, Helicobacter pylori,4Mycobacterium tuberculosis), virus (Hepatitis B virus (HBV), Herpes simplex virus (HSV)5), parasites6 and fungi.7,8 In some cases, the causal relationship was not proved and the exact role and pathogenesis of mast cell activation by infections remain unclear.2 However, the temporal relationship between infections and urticaria beginning as well as urticaria remission or improvement after treatment of a coincident infection, support this link.2
In this study, we hypothesized that streptococcal tonsillitis (ST) could be a primary cause of CU or acute recurrent urticaria (ARU).
Materials and methodsThis retrospective study included 14 outpatients observed in the Urticaria and Allergic Skin Disease Consultation of our Immunoallergy Department, between January 2000 and December 2009, with CU or ARU according to EAACI/GA2LEN/EDF/WAO position paper definition9 and clinical and/or laboratorial suspicion of an aetiopathogenic link with ST.
We looked for the clinical history (symptoms, exacerbation factors – namely the existence of a temporal relationship between urticaria episodes and tonsillitis – and clinical evolution), the objective examination and the laboratorial study, including markers of streptococcal infection (anti-streptolysin O title (ASOT)) and other tests: differential blood count, renal and hepatic function, complement fractions, circulating immune complexes (CIC), serum immunoglobulins, viral serologies (Hepatitis A, B and C Virus, HSV, Cytomegalovirus and Epstein–Barr virus), screening test for syphilis (VDRL), antinuclear and anti-neutrophil cytoplasmic antibodies, thyroid hormones and auto-antibodies, rheumatoid factor, skin prick tests (SPT) of the most prevalent aeroallergens of the region, stool for ova and parasites and thorax X-ray.
Three groups were defined according to clinical evolution: spontaneous resolution of urticaria, resolution after tonsillectomy and still symptomatic.
ResultsThe 14 patients (64.3% female) included have a current age of 25.6±8.3 years. At the beginning of urticaria, patients had 17.1±8.0 years, with 3.4±3.0 years of disease evolution until the first consultation. Relevant data of the clinical history and oropharyngeal examination are presented in Table 1. A clinical relationship between urticaria episodes and underling infections was denied by all patients at the first consultation. Later, 71.4% of patients recognised that urticaria exacerbations were coincident with tonsillitis. Additionally to anti-histamines, antibiotic was prescribed at the first signs of ST, resulting in less severe exacerbations of urticaria.
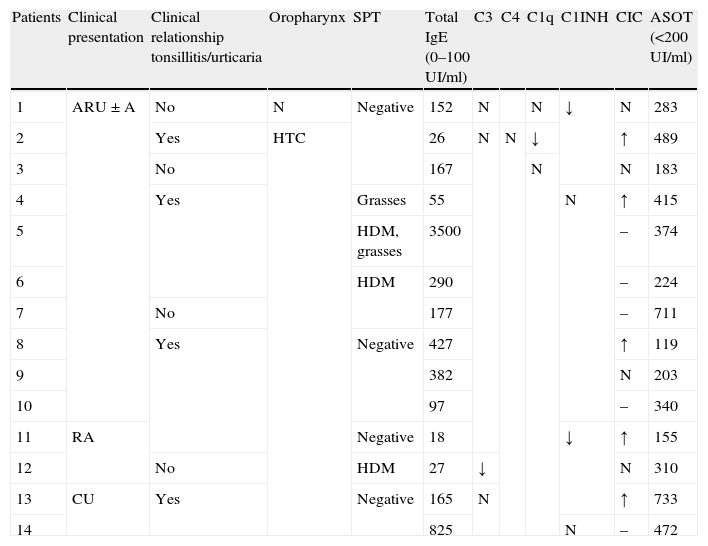
Relevant data of the clinical history, oropharyngeal examination and immunoallergological study.
| Patients | Clinical presentation | Clinical relationship tonsillitis/urticaria | Oropharynx | SPT | Total IgE (0–100UI/ml) | C3 | C4 | C1q | C1INH | CIC | ASOT (<200UI/ml) |
| 1 | ARU±A | No | N | Negative | 152 | N | N | ↓ | N | 283 | |
| 2 | Yes | HTC | 26 | N | N | ↓ | ↑ | 489 | |||
| 3 | No | 167 | N | N | 183 | ||||||
| 4 | Yes | Grasses | 55 | N | ↑ | 415 | |||||
| 5 | HDM, grasses | 3500 | – | 374 | |||||||
| 6 | HDM | 290 | – | 224 | |||||||
| 7 | No | 177 | – | 711 | |||||||
| 8 | Yes | Negative | 427 | ↑ | 119 | ||||||
| 9 | 382 | N | 203 | ||||||||
| 10 | 97 | – | 340 | ||||||||
| 11 | RA | Negative | 18 | ↓ | ↑ | 155 | |||||
| 12 | No | HDM | 27 | ↓ | N | 310 | |||||
| 13 | CU | Yes | Negative | 165 | N | ↑ | 733 | ||||
| 14 | 825 | N | – | 472 |
SPT – skin prick test; CIC – circulating immune complexes; ASOT – anti-streptolysin O title; ARU±A – acute recurrent urticaria with or without angioedema; RA – recurrent angioedema; CU – chronic urticaria; HTC – hypertrophic tonsils with crypts; N – normal; HDM – house dust mites.
Some relevant data of the immunoallergological study are presented in Table 1, with the remaining normal study.
The clinical evolution is described in Table 2. Demographical and clinical differences between the three groups defined according to clinical evolution, are presented in Table 3.
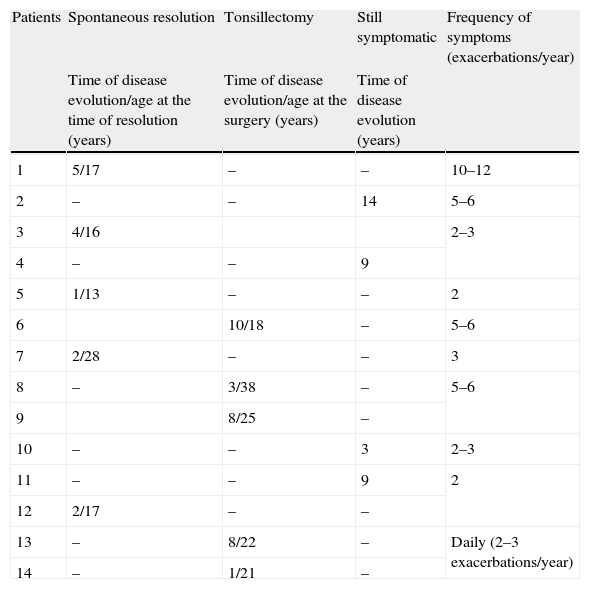
Clinical evolution of urticaria and/or angio-oedema.
| Patients | Spontaneous resolution | Tonsillectomy | Still symptomatic | Frequency of symptoms (exacerbations/year) |
| Time of disease evolution/age at the time of resolution (years) | Time of disease evolution/age at the surgery (years) | Time of disease evolution (years) | ||
| 1 | 5/17 | – | – | 10–12 |
| 2 | – | – | 14 | 5–6 |
| 3 | 4/16 | 2–3 | ||
| 4 | – | – | 9 | |
| 5 | 1/13 | – | – | 2 |
| 6 | 10/18 | – | 5–6 | |
| 7 | 2/28 | – | – | 3 |
| 8 | – | 3/38 | – | 5–6 |
| 9 | 8/25 | – | ||
| 10 | – | – | 3 | 2–3 |
| 11 | – | – | 9 | 2 |
| 12 | 2/17 | – | – | |
| 13 | – | 8/22 | – | Daily (2–3 exacerbations/year) |
| 14 | – | 1/21 | – |
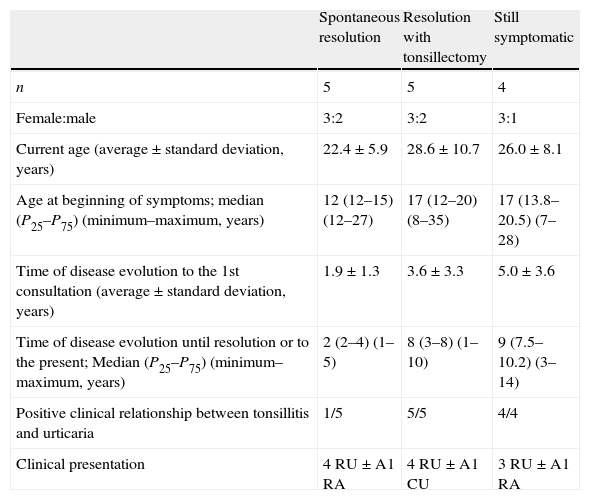
Demographical and clinical differences between the three groups of patients, defined according to clinical evolution.
| Spontaneous resolution | Resolution with tonsillectomy | Still symptomatic | |
| n | 5 | 5 | 4 |
| Female:male | 3:2 | 3:2 | 3:1 |
| Current age (average±standard deviation, years) | 22.4±5.9 | 28.6±10.7 | 26.0±8.1 |
| Age at beginning of symptoms; median (P25–P75) (minimum–maximum, years) | 12 (12–15) (12–27) | 17 (12–20) (8–35) | 17 (13.8–20.5) (7–28) |
| Time of disease evolution to the 1st consultation (average±standard deviation, years) | 1.9±1.3 | 3.6±3.3 | 5.0±3.6 |
| Time of disease evolution until resolution or to the present; Median (P25–P75) (minimum–maximum, years) | 2 (2–4) (1–5) | 8 (3–8) (1–10) | 9 (7.5–10.2) (3–14) |
| Positive clinical relationship between tonsillitis and urticaria | 1/5 | 5/5 | 4/4 |
| Clinical presentation | 4 RU±A1 RA | 4 RU±A1 CU | 3 RU±A1 RA |
RU – recurrent urticaria; A – angio-oedema; CU – chronic urticaria.
An average of 109 patients by year are observed in our Urticaria and Allergic Skin Disease Consultation, with a prevalence of infectious urticaria of about 4.6%.10
In these 14 patients, several causes of urticaria and/or angioedema were excluded, namely: allergy, physical urticaria, autoimmunity (including autoimmune thyropathy), hereditary angioedema and other infections (parasitosis, viral infections, bacterial processes such as sinusitis and dental infections).
Given the high prevalence of CU/ARU and ST, they can coexist in the same patient independently. Nevertheless, our study supports an eventual causal relationship between them, by markers of streptococcal infection in all patients (clinical symptoms and/or presence of hypertrophic tonsils with crypts and/or high ASOT), the perception of a clinical relationship between tonsillitis and urticaria (71.4% of patients), the decrease of urticaria severity with early antibiotherapy to tonsillitis and urticaria resolution after tonsillectomy in the five patients, with 6.0±3.8 years of disease evolution.
Besides the controversial relationship between infections and CU, namely tonsillitis, it has been described in the literature.11 In 1967, Buckley et al. described 16 children with CU, the main feature of 15 being recurrent upper respiratory infection, pharingitis, tonsillitis, sinusitis and otitis, often by Streptococci or Staphylococci. These authors observed that remission of urticaria was frequently noted following antibiotherapy.12 Buss et al.13 identified tonsillitis or sinusitis in 50% of patients with CU and high ASOT have been described in 10–42%.2
There is a wide variation in the reported prevalence of infections as a cause of CU. In a systematic review about the underlying causes of physical and chronic urticaria and angioedema, eight studies reported infections in 11–31% of the patients, whereas 11 reported them in 0–6%.14 Considering this discrepancy, the EAACI/GA2LEN/EDF/WAO guidelines9 make no definitive recommendations regarding the role of infections in urticaria, although testing for infectious diseases is suggested as an additional investigation of chronic spontaneous urticaria, and infections treatment is recommended in the management of urticaria.11 Indeed, in our study, the two patients with CU reported a temporal relationship between CU exacerbations and tonsillitis, and tonsillectomy was coincident with CU resolution. In our opinion, ST must always be looked for, even in the absence of a clinical relationship between tonsillitis and urticaria, since chronic ST can be asymptomatic, working as an occult infection. This fact can explain the absence of this clinical relationship in some patients.
With regard to the aetiopathogenic mechanism linking tonsillitis and urticaria, a laboratorial pattern was not found in our study. However, the laboratorial findings of patient 2 are similar to that described for acquired angio-oedema, with complement consumption through C1q activation by CIC. Laboratorial data of patients 11 and 13 are also suggestive of complement activation mediated by CIC, supporting the same mechanism. This mechanism could also be implicated in those patients with isolated high CIC, admitting that the normal levels of complement fractions could be explained by the timing of the study, since not all patients were studied during an active phase of urticaria. From the remaining six patients, CIC evaluation was not performed in five of them. There is probably more than one mechanism linking streptococcal infection and urticaria, which could be present alone or together. Indeed, complement direct activation from Streptococcus is described.
A potential link between persistent infection and the development of autoimmune mechanisms in CU has been discussed. In CU, positivity of autologous serum skin test has been associated with H. pylori infection and in some patients this test becomes negative after Helicobacter eradication.15 The persistence of streptococcal infection has been associated with autoreactivity,16 leading to rheumatic fever and rheumatic heart disease, which result from autoimmune humoral and cell mediated reactions due to the molecular mimicry between haemolytic streptococcus group A antigens and host proteins.17 The prevalence of infections in CU is similar to the general population; however, patients with CU may differ in their immune response to infections, or may develop infection-induced autoreactivity/autoimmunity.2 Autologous serum skin test and 13C urea breath test were not performed in these patients since a plausible cause for urticaria was found.
Underlying genetic predisposition is also proposed by some authors as a possible explanation for the development of CU in response to bacterial infection, once associations with major histocompatibility complex II have been shown for CU.18
Since in these patients urticaria seems to be associated with ST, its early treatment and frequency decrease is desirable. As referred before, prompt antibiotherapy improved exacerbation severity but the long-term clinical evolution was discrepant between patients (Table 2).
There are well defined criteria to tonsillectomy that do not include this one alone.19 In our study, tonsillectomy was curative in the five patients. However, given this restricted number and the fact that some experienced spontaneous resolution, we cannot assess the effectiveness of tonsillectomy in order to recommend it to all patients with this clinical picture. Probably, there are prognostic factors that would be important to find out.
We observed that patients with spontaneous resolution experienced a shorter time of disease evolution. A clinical relationship between tonsillitis and urticaria was reported by all patients who did not have spontaneous resolution, as opposed to 1/5 in the group of spontaneous resolution. We can speculate that the longer time of disease evolution offers more opportunities to perceive this relationship.
We propose that those patients who accomplish tonsillectomy criteria should be promptly referenced to an ENT consultation; the others should be followed during the first two years of disease evolution and if a spontaneous resolution does not occur, tonsillectomy should be considered.
ConclusionAlthough randomised controlled trials are lacking, there is increasing evidence that recurrent or persistent infections may have a primary role in CU or ARU. Our study also corroborates this relationship, at least in some patients.
Our laboratorial data suggest complement activation as the main immunopathogenical mechanism of mastocyte activation, besides the pathogenesis of CU or ARU in any given patient may be multifactorial.
The prognosis of urticaria is favourable when the aetiology is established. Therefore, it should be treated by the identification and elimination of underlying causes whenever possible. Our study encourages the investigation of tonsillitis in these otherwise idiopathic patients, especially until young adulthood, and even in the absence of any symptoms.
Larger studies are warranted to define prognostic factors of longstanding disease in order to better select those patients who would benefit from tonsillectomy.
Conflict of interestThe authors have no conflict of interest to declare.