Exposure to allergens in early life may predispose subjects to develop allergies and diseases related to allergic sensitisation.
ObjectiveTo determine the association between month of birth and atopic sensitisation in adult Turkish patients with rhinitis and/or asthma using the diagnostic method of skin prick tests.
MethodsThis prospective cross-sectional study included all adult patients who underwent skin prick testing with rhinitis and asthma from November 2009 to June 2010. Sensitisation was categorised as any sensitisation, pollen sensitisation, and house dust mite sensitisation. Multivariate logistic regression model was employed with the primary predictor being month of birth. Diagnosis (asthma, rhinitis and both), age, gender and family history of atopy were considered as potential confounders in the model. The associations were presented with both unadjusted and adjusted odds ratios (OR) and their 95% confidence interval (CI).
ResultsA total of 616 subjects were evaluated. Three-hundred and forty-one subjects had sensitisation to allergens according to skin prick tests. Analyses showed that subjects born in September were less likely to have documented skin test positively with pollen sensitisation [0.27 (0.09–0.84), p=0.023].
ConclusionThe results support the hypothesis that being born at the end of the pollen season may protect subjects from pollen sensitisation.
It is well known that allergic diseases occur as a consequence of genetic and environmental interactions. Since allergic diseases have been increasing in recent decades, the impact of environmental factors on the prevalence of allergic diseases has become a growing concern throughout the world. Improving our understanding of the causes of increase in allergic diseases may help to improve treatments and preventive strategies.
The first month of life is considered as vulnerable period in the development of atopic diseases. Earlier reports have demonstrated a positive association between sensitisation to pollens and month of birth during March–May and January–May.1,2 Other studies have identified a trend towards an increased risk for subjects born in the grass pollen season (May–June) to develop grass pollen allergy in children.3,4 Subjects born in the pollen season (March–April) have been shown to have increased risk of being sensitised to birch pollen.3 Another study also supported an increased risk of developing allergic skin sensitisation for grass pollen in those patients born in February, May and June4 and an association between allergic diseases such as hay fever and month of birth.4 A protective effect has been reported for certain months of birth: September for allergic sensitisation; October for asthma; and November for hay fever.4 There are less data on the association of birth date and allergic sensitisation in adult patients. One study reported that month of birth was associated with sensitisation to certain pollens in seasonal allergic rhinitis and asthmatic adult patients.5
The aim of the present study was to determine the association between month of birth and atopic sensitisation in adult Turkish patients with respiratory diseases (rhinitis and asthma) using the diagnostic method of skin prick tests. Turkey is located in both Asia and Europe, has coasts on the Mediterranean, Black Sea and the Aegean Sea. Generally, Turkey's subcontinental Mediterranean-type climate is home to diverse fauna and associated pollens. We hypothesised that the association between birth month and allergen sensitisation would be present in adults in this environment yet show a different seasonal pattern.
MethodsThe study was a prospective cross-sectional study. All adult patients who underwent skin prick testing with rhinitis and/or asthma in our outpatient clinic from November 2009 to June 2010 who provided informed consent were included in the study.
Inclusion Criteria:
- 1-
All patients with rhinitis and asthma who underwent skin prick test with inhalant allergens.
- 2-
The patients for whom we have birth date.
Exclusion Criteria:
- 1-
Skin prick tested patients for food allergy, drug allergy and bee allergy.
- 2-
Patients with chronic systemic diseases causing malnutrition.
- 3-
Patients with dermographism or any other skin disease which may affect skin prick test results.
- 4-
Patients who were using antihistamines, antileukotrienes, antidepressants and systemic steroids which may affect the skin prick test results.
- 5-
Active smokers
Asthma was diagnosed according to the definition of the American Thoracic Society.6 Atopic asthma was defined as an asthma diagnosis with any sensitisation to skin prick test. Rhinitis patients were defined by documentation in the medical history of the following symptoms: nasal itching, rhinorrhea, sneezing, and nasal obstruction. The diagnosis of hay fever was made on the basis of seasonal characteristics of the rhinitis symptoms and sensitisation to pollens. Perennial allergic rhinitis (PAR) was defined as rhinitis diagnosis with perennial symptoms and sensitisation to allergens. Family history of any of the allergic diseases, including asthma, allergic rhinitis, eczema and atopic dermatitis in a first-degree relative was used to define atopic family history.
Skin prick test (SPT)Skin prick tests were performed using a common panel including: Dermatophagoides pteronyssinus, Dermatophagoides farinae, grass, tree and weed pollens and moulds (including Alternaria, Cladosporium, Aspergillus, Penicillium) allergen extracts (ALK, Spain). Positive and negative controls were histamine and phenolated glycerol saline, respectively. A mean wheal diameter of 3mm or greater than that obtained with control solution was considered positive.
Ethical approval of the Eskişehir Osmangazi University Institutional Ethical Board was obtained and all patients gave informed consent for the study.
Statistical analysisDescriptive statistics included frequencies, percent distributions, mean and standard deviation for normally distributed data (age). Comparisons of age between groups were made using the One-way Anova test and the Mann–Whitney U test. Between group comparisons for categorical variables were made using the Chi-square test. Multivariate logistic regression analysis was employed to assess the association between month of birth and skin sensitisation and allergic diseases. Sensitisation was categorised as any sensitisation, pollen sensitisation, or house dust mite sensitisation. The reference month was January. Diagnosis (asthma, rhinitis and both), age, gender (male was reference) and family history of atopy were considered as potential confounders and included in the regression model. We employed the Hosmer–Lemeshow test to assess goodness of fit of our final logistic regression model. The strength of the relationship of sensitisation and month of birth was evaluated by calculating adjusted odds ratios (OR) and their 95% Confidence Interval (CI) in respect to reference month. Age was analysed as a continuous covariate. All other variables were coded as dichotomous categorical covariates. A two-tailed p value of less than 0.05 was considered as statistically significant. The data were analysed with SPSS computer program for Windows, version 15.0.
ResultsSix-hundred and sixteen subjects were enrolled in this study. The mean age was 39.3±13.7 years [median 38 (range: 16–75)]. Four-hundred and twenty-eight subjects were females (69%) (mean age was 40.9±13.7 years old) and 188 subjects were males (mean age was 35.5±13.7 years old). Three-hundred and forty-one patients had allergic sensitisation according to skin prick test results (56%). One-hundred and sixty-seven patients had sensitisation to pollens, 99 patients had sensitisation to mites, 67 patients had sensitisation to both and eight patients had sensitisation to moulds. Two-hundred and thirty-nine subjects had asthma, 234 subjects had rhinitis and 143 subjects had asthma plus rhinitis. Two-hundred and seventy-three patients (44.3%) declared family history of atopic disease.
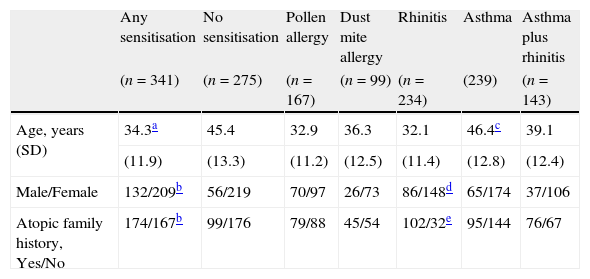
Two-hundred and thirty-four patients had rhinitis, 239 had asthma and 143 had asthma plus rhinitis (Table 1). One hundred and sixty-six subjects had atopic asthma, 95 had hay fever and 82 had PAR. Atopic subjects had younger age (34.3±11.9 versus 45.4±13.3 years, p<0.0001) and had more frequent family history of atopic disease (χ2=14.0, p<0.0001) than non-atopic subjects (Table 1). Distribution of gender and atopic family history were different between sensitised and non-sensitised subjects (p<0.0001 and p<0.0001 respectively) (Table 1).
Characteristics of study subjects.
| Any sensitisation | No sensitisation | Pollen allergy | Dust mite allergy | Rhinitis | Asthma | Asthma plus rhinitis | |
| (n=341) | (n=275) | (n=167) | (n=99) | (n=234) | (239) | (n=143) | |
| Age, years (SD) | 34.3a | 45.4 | 32.9 | 36.3 | 32.1 | 46.4c | 39.1 |
| (11.9) | (13.3) | (11.2) | (12.5) | (11.4) | (12.8) | (12.4) | |
| Male/Female | 132/209b | 56/219 | 70/97 | 26/73 | 86/148d | 65/174 | 37/106 |
| Atopic family history, Yes/No | 174/167b | 99/176 | 79/88 | 45/54 | 102/32e | 95/144 | 76/67 |
SD, standard deviation.
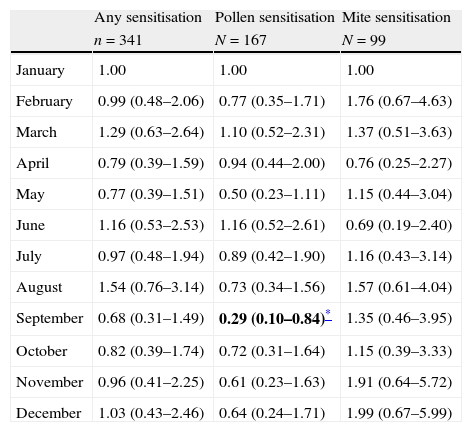
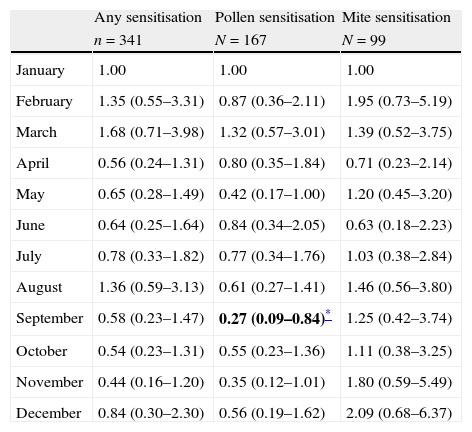
Subjects born in September were less likely to have documented positive skin test result to pollens in unadjusted multivariate regression analysis [OR: 0.29, 95% CI=(0.10–0.84), p=0.023) (Table 2). The adjusted associations between sensitisation to allergens and month of birth in multivariate regression analysis showed that subjects born in September were less likely to have documented skin test positively with pollen sensitisation [OR=0.27, 95% CI=(0.09–0.84), p=0.023] (Table 3). The assessment of goodness of fit using the Hosmer–Lemeshow test noted no significant difference (p=0.10), suggesting adequate model fit. The distribution of sensitisation to pollens according to month of birth is presented in Fig. 1. Pollen counts reach their highest level in May whereas they are absent between November and January in Eskişehir atmosphere in a study from 1996 (Fig. 2).7
Unadjusted associations between sensitisation to allergens and month of birth in multivariate regression analysis (results are given as OR: odds ratio and CI: confidence interval).
| Any sensitisation | Pollen sensitisation | Mite sensitisation | |
| n=341 | N=167 | N=99 | |
| January | 1.00 | 1.00 | 1.00 |
| February | 0.99 (0.48–2.06) | 0.77 (0.35–1.71) | 1.76 (0.67–4.63) |
| March | 1.29 (0.63–2.64) | 1.10 (0.52–2.31) | 1.37 (0.51–3.63) |
| April | 0.79 (0.39–1.59) | 0.94 (0.44–2.00) | 0.76 (0.25–2.27) |
| May | 0.77 (0.39–1.51) | 0.50 (0.23–1.11) | 1.15 (0.44–3.04) |
| June | 1.16 (0.53–2.53) | 1.16 (0.52–2.61) | 0.69 (0.19–2.40) |
| July | 0.97 (0.48–1.94) | 0.89 (0.42–1.90) | 1.16 (0.43–3.14) |
| August | 1.54 (0.76–3.14) | 0.73 (0.34–1.56) | 1.57 (0.61–4.04) |
| September | 0.68 (0.31–1.49) | 0.29 (0.10–0.84)* | 1.35 (0.46–3.95) |
| October | 0.82 (0.39–1.74) | 0.72 (0.31–1.64) | 1.15 (0.39–3.33) |
| November | 0.96 (0.41–2.25) | 0.61 (0.23–1.63) | 1.91 (0.64–5.72) |
| December | 1.03 (0.43–2.46) | 0.64 (0.24–1.71) | 1.99 (0.67–5.99) |
Adjusted associations between sensitisation to allergens and month of birth in multivariate regression analysis (results are given as OR: odds ratio and CI: confidence interval).
| Any sensitisation | Pollen sensitisation | Mite sensitisation | |
| n=341 | N=167 | N=99 | |
| January | 1.00 | 1.00 | 1.00 |
| February | 1.35 (0.55–3.31) | 0.87 (0.36–2.11) | 1.95 (0.73–5.19) |
| March | 1.68 (0.71–3.98) | 1.32 (0.57–3.01) | 1.39 (0.52–3.75) |
| April | 0.56 (0.24–1.31) | 0.80 (0.35–1.84) | 0.71 (0.23–2.14) |
| May | 0.65 (0.28–1.49) | 0.42 (0.17–1.00) | 1.20 (0.45–3.20) |
| June | 0.64 (0.25–1.64) | 0.84 (0.34–2.05) | 0.63 (0.18–2.23) |
| July | 0.78 (0.33–1.82) | 0.77 (0.34–1.76) | 1.03 (0.38–2.84) |
| August | 1.36 (0.59–3.13) | 0.61 (0.27–1.41) | 1.46 (0.56–3.80) |
| September | 0.58 (0.23–1.47) | 0.27 (0.09–0.84)* | 1.25 (0.42–3.74) |
| October | 0.54 (0.23–1.31) | 0.55 (0.23–1.36) | 1.11 (0.38–3.25) |
| November | 0.44 (0.16–1.20) | 0.35 (0.12–1.01) | 1.80 (0.59–5.49) |
| December | 0.84 (0.30–2.30) | 0.56 (0.19–1.62) | 2.09 (0.68–6.37) |
Distribution of atmospheric pollen counts for each month in Eskişehir.7
Results of the present study are in accordance with previous studies showing association between month of birth and sensitisation to pollen allergens. The most striking result was the decreased risk of having sensitisation to pollens with being born in September, as in the study of Wijst et al.4 Worldwide variability of prevalence of asthma and allergic diseases is not explained only by genetic background. It is well known that allergic diseases occur as a consequence of genetic and environmental interaction. Epidemiological studies on environmental risk factors mostly focused on micro environmental characteristics such as housing and life style, which are more preventable. Among environmental factors, outdoor environmental factors have been shown to be risk factors for allergic diseases and asthma.8,9
Exposure to a certain allergen causes sensitisation in a genetically predisposed person. This relationship shows a positive dose response. A number of studies support the hypothesis that allergen levels in inhaled air especially in the home are associated with increased risk of allergic sensitisation.10–12 High levels of allergen exposure also may lead not only to the development of respiratory disease but account for the severity of respiratory disease.13,14 The age of exposure was also investigated for its effect on the sensitisation and it was found that even in utero exposure affects the sensitisation.15 The dose and effect relationship seems to be more prominent when exposure occurs during early years of childhood.16 Pollens are the major outdoor allergen and their outdoor levels have been found to be associated with sensitisation to a particular pollen17 and symptoms of allergic disease.18 It has been suggested that early postnatal exposures are crucial for the development of allergic sensitisation. Most of the previous reports showed a positive association between sensitisation to pollens and month of birth during the pollen season.1–4 However, some reports published a protective effect of month of birth on sensitisation, such as low sensitisation to pollens with being born in September4 and low sensitisation to moulds with being born in April, May and December.2 This could be due to higher sensitisation throughout the remaining months due to local environmental effects thus leading to months that appear to be protective. Exposure to certain pollens in the early months after birth is considered important for sensitisation to the pollens. Previous reports suggested that being born a few months before the peak of pollen season or exposure to pollen during the first 6 months were important determinants for subsequent pollinosis in children.19,20 Being born at the end of the pollen season may allow protection from exposure to pollens during the first months of post natal life which may cause decreased sensitisation to pollens.
Turkey is located in the Northern hemisphere and has a subcontinental Mediterranean-type climate that allows growing different types of plants, causing a variety of pollens dispersed in the air. Generally, the pollen season occurs from March to October with a more intense period between April and June.7,21 The peak of pollen counts is found in May.7,21 In the present study, we performed a separate analysis for those patients with dust mite sensitisation with multivariate analysis. Due to the rarity of sensitisation (only eight subjects who had sensitisation to moulds), a separate analysis was not performed related to mould sensitisation. Dust mite allergen exposure occurs mainly in the home environment in the first years of life, thus sensitisation may not be affected by the month of birth unless time indoors is significantly impacted by season. In our analysis, we found no association with dust mite sensitisation and month of birth. Sensitisation to indoor allergens is likely to vary in a certain geographical area from home to home modified significantly by climate.
In Turkey, that the pollen season ends in October, potentially explains our results (Fig. 2). Based on geography, our findings may be due to lower or no exposure to pollen allergens during post-natal period (after September). Other studies have noted this potential protective effect seen in subjects born in September in the Northern hemisphere.4
There are a number of important limitations in this study. Due to the multifactorial nature of allergic diseases, unmeasured confounders could account for our findings. However, very few variables are associated with month of birth (true confounders). Second, we were unable to determine all life-long allergen exposure for each individual. Subjects could have grown up in other countries or regions with very different aeroallergen exposure. However, subjects in the present study were born and raised in this region (Eskisehir and neighbouring cities) with very limited immigration or emigration. Last, this study may not be generalised to those patients who do not have access to referral services or have limited symptomatology due to referral bias. Given the healthcare system in our region, this is a limited population.
The strengths of this study include the clear phenotypic description of patients with specific respiratory diseases (asthma and/or rhinitis). This is the first study showing association of atopy and birth date in Turkey. Our results support the hypothesis that the first few months of life constitute a sensitive period, during which protection from exposure to pollen allergens may be associated with decreased sensitisation to pollens. The results of this study could assist in identifying high risk groups for atopic sensitisation which in turn will guide future preventive services. Others have suggested that prevalence of sensitisation to pollens could be reduced approximately 20% by eliminating early pollen contacts.22 It is likely that among individuals who are genetically susceptible to develop pollen sensitisation, environmental pollen exposure in the early months of life influences the development of the sensitisation. Planning the birth date for babies is a possible recommendation for parents with a strong family history of pollen allergy.
ConclusionSensitisation to pollens might be influenced by environmental effects in the early months of life in genetically susceptible individuals.
Conflict of interestThe authors have no conflict of interest to declare.
The present study was planned during the Methods in Epidemiologic, Clinical & Operations Research (MECOR) course. The authors thank the MECOR course programme, its mentors and its founder Sonia Buist.