Bronchial hyperresponsiveness is the pathogenic basis of asthma, and measurement of its intensity is investigated using the methacholine provocation test, which not only and particularly evaluates the reduction in FEV1 (PD20) but also takes forced mid-expiratory flow or FEF25–75 (PD40) into account. The present study aims to evaluate the usefulness of both parameters.
Material and methodsProvocation testing was carried out in 151 patients between 7 and 22 years of age diagnosed with asthma, tracheobronchitis and/or rhinitis, using a short method that allows quantification of the methacholine administered. The subjects were divided into three groups according to the amount of methacholine needed to obtain the mentioned parameters (group 1: ≤1000μg; group 2: 1001–2000μg; group 3: ≥2001μg).
ResultsGreater variability was recorded for FEF25–75 than for FEV1. Paired comparison among the three groups for FEV1 proved significant, in the same way as for FEF25–75 between groups 2 and 3, and 1 and 3, but not between groups 1 and 2. Calculation was made of the amount of methacholine required to obtain PD20 and PD40 from the same dose. Only the significant differences corresponded to the comparison of group 1 versus the rest, with no differences between the means of the total mean values.
ConclusionsThe utility of PD20 is more evident, considering the variability of PD40; the latter may be useful in patients with rhinitis or tracheobronchitis when PD20 proves scantly demonstrative.
One of the pathogenic bases of asthma is enhanced bronchial smooth muscle contractility (bronchial lability or bronchial hyperresponsiveness [BHR]), which is the cause of the acute breathing difficulty (dyspnoea) episodes that characterise the disease. Muscle contractility varies from one asthmatic patient to another, and its intensity is measured through the inhalation of methacholine (methacholine test), which induces smooth muscle contraction. Successive methacholine doses are administered until the forced expiratory volume in 1s (FEV1) is seen to decrease 20% (PD20). With that same amount of methacholine, the value of the midzone of the curve used to assess flow (forced mid-expiratory flow: FEF25–75) decreases considerably more (approximately 40%) (PD40), and this value corresponds to the thinner portions of the bronchial tree (small airways).
Although PD20 is the value usually considered for determining the degree of BHR,1 recent studies underscore the greater interest of knowing the reduction of FEF25–75, which informs of the lability of the small airways, which are more intimately implicated in the pathogenesis of the dyspnoea episodes.2–4 The present study was carried out to determine whether there are significant differences between the two parameters (PD20 and PD40) in relation to different degrees of sensitivity to methacholine, and whether one parameter or the other is more useful for assessing the degree of bronchial responsiveness and its relationship with the small airways.
Material and methodsPatientsBronchoconstriction testing with methacholine was carried out in a total of 151 patients between 7 and 22 years of age, mostly diagnosed with asthma and/or allergic rhinitis, and also including some children in whom the existence of atopic problems could not be demonstrated due to the recording of negative allergy tests and normal serum IgE levels, but who suffered recurrent respiratory processes diagnosed as wheeze bronchitis.5 In most of the patients, the test was carried out as a complement to the initial evaluation of the disorder, at the time of the diagnosis.
Bronchial responsiveness varies from one patient to another – a circumstance that may be related to the diagnosis or to the severity of the process. Therefore, and based on the methacholine dose needed to reach PD20, the patients were evaluated separately, divided into three groups: group 1: ≤1000μg, 62 patients; group 2: 1001–2000μg, 31 patients; and group 3: ≥2001μg, 58 patients. The mean patient age in the three groups was similar. Table 1 reports the patient characteristics.
Patient characteristics.
| N°. patients | Dominant diagnosis | Initial mean value (baseline) | ||||||
| Sex | Asthma | Rhinitis | Rhinitis/bronchitis | FEV1/FVC% | FEV1 (l) | FEF25–75 (l/s) | ||
| Group 1: ≤1000μg | 62 | V:40H:22 | 54 | 8 | 80 | 2.27 | 2.2 | |
| Group 2: 1001–2000μg | 31 | V:21H:10 | 26 | 5 | 87 | 2.35 | 2.4 | |
| Group 3: ≥2001μg | 58 | V:44H:14 | 24 | 8 | 26 | 88 | 2.83 | 3.0 |
An abbreviated method has been used in which the aerosol is inhaled during inspiration, allowing quantification of the administered methacholine dose, from a single concentration of the drug.6,7 The patients were asymptomatic at the time of provocation with methacholine; had received no bronchodilatory or anti-inflammatory medication for at least two days before testing; and presented normal respiratory function, with FEV1/FVC>70.1 The methacholine formulation (Provocholine®, Roche) was diluted 1/100 with physiological saline, yielding concentrations of 10mg/ml. Spirometry was carried out with the Vicatest Spimco (Mijnhardt, The Netherlands) before testing and again 2min after each of the inhalations (Mediprom FDC 88 dosimeter, Paris, France). Fitting a mouthpiece to the nebuliser (De Vilbiss 5610 D), the patient was instructed to breathe normally, and after a forced expiration performed a maximum inspiration (1–2s), followed by a 3-s apnoea phase and then gentle expiration.1,8 The decrease in FEV1 was estimated from the value of this parameter obtained after the inhalation of physiological saline solution prior to the start of the test (baseline). At first inhalation we administered 100μg (0.5μmol) of methacholine and then repeatedly administered 200μg (cumulative dosage: 300μg, 500μg, 700μg, 900μg, etc.). The test ended when FEV1 dropped approximately 20% (PD20) – this value being posteriorly calculated from the dose–response curve. Administration was suspended if this decrease in FEV1 was not reached with the maximum cumulative dose of 2100μg. The amount of methacholine needed to obtain both parameters in the three groups was also verified in order to assess the possible value.
Statistical analysisThe Wilcoxon signed-rank test was used to compare pairs of related samples and explore differences between them. This was taken to be the most appropriate option, since it is a non-parametric test for comparing paired groups, calculating the differences between each pair of data. The Mann–Whitney U-test in turn was used to compare the values of FEV1 and FEF25–75 between the different groups, since in this case the individuals were different and thus the values were independent.
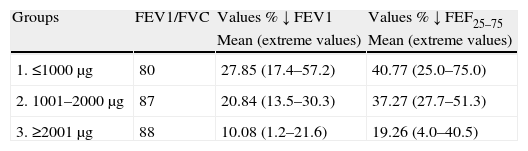
ResultsBased on the maximum methacholine doses administered for calculating PD20 and PD40, we evaluated the percentage decrease in both parameters (FEV1/FVC and FEF25–75) starting from the baseline values of the patients before the test (Table 2). This table reports the mean percentage decrease in the values for the total patients included in each group. Paired comparison among the three groups for FEV1 proved significant (p<0.01 or p<0.001), in the same way as for FEF25–75 between groups 2 and 3, and 1 and 3, but not between groups 1 and 2 (p>0.13). Likewise, comparison of the mean total values proved very significant (FEV1: 19.59; FEF25–75: 32.43). In all patients FEV1/FVC was >70 as required for this test.5 The best variability was noticed in the three groups of extreme values of PD40 in contrast with PD20.
Comparison of the maximum/minimum values and means of the reductions in FEV1 and FEF25–75.
| Groups | FEV1/FVC | Values % ↓ FEV1 | Values % ↓ FEF25–75 |
| Mean (extreme values) | Mean (extreme values) | ||
| 1. ≤1000μg | 80 | 27.85 (17.4–57.2) | 40.77 (25.0–75.0) |
| 2. 1001–2000μg | 87 | 20.84 (13.5–30.3) | 37.27 (27.7–51.3) |
| 3. ≥2001μg | 88 | 10.08 (1.2–21.6) | 19.26 (4.0–40.5) |
| Mean of the three groups: 19.59 | Mean of the three groups: 32.43 | |||||
| Statistical significance between the total mean values of FEV1 (19.59) and FEF (32.43); p<0.001. | ||||||
| Statistical differences of the mean values between groups | ||||||
| Groups → | 1–2 | 2–3 | 1–3 | 1–2 | 2–3 | 1–3 |
| Statistical significance | p<0.01 | p<0.001 | p<0.001 | p>0.13 | p<0.001 | p<0.001 |
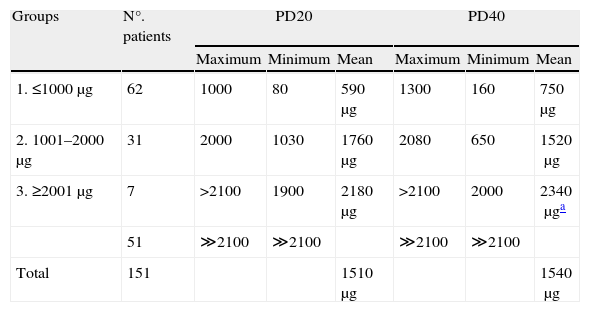
The amount of methacholine needed to reach PD20 (the point at which testing was stopped) and PD40 based on the same dose was also calculated. The only significant differences corresponded to the comparison of group 1 versus the rest of the groups (p<0.001), with no significant differences between the means of the total mean values (p>0.85) (Table 3). In seven of the patients in group 3 at least one of the two parameters was obtained with the dose equal to or lower than the maximum administered amount – the other parameter being theoretically calculated according to this dose. In the other 51 patients of this group, doses in excess of 2100μg would have been required to reach these values; as a result, these cases were considered negative or scantly hyper-responsive, and excluded from the calculus. This group comprised most of the patients diagnosed with rhinitis or tracheobronchitis, without episodes of dyspnoea, while others were diagnosed with mild asthma. In this same group, on comparing the patients predominantly diagnosed with asthma (n=29) versus those diagnosed with rhinitis (n=24), a statistically significant difference was observed in the percentage reduction in values for PD40 (asthma ↓24%; rhinitis ↓16%: p<0.001), but not for PD20 (asthma ↓11%; rhinitis ↓9%: p>0.09). Between boys and girls we likewise recorded no significant differences in any of the three groups, in relation to both parameters.
Methacholine required to reach PD20 and PD40.
| Groups | N°. patients | PD20 | PD40 | ||||
| Maximum | Minimum | Mean | Maximum | Minimum | Mean | ||
| 1. ≤1000μg | 62 | 1000 | 80 | 590μg | 1300 | 160 | 750μg |
| 2. 1001–2000μg | 31 | 2000 | 1030 | 1760μg | 2080 | 650 | 1520μg |
| 3. ≥2001μg | 7 | >2100 | 1900 | 2180μg | >2100 | 2000 | 2340μga |
| 51 | ≫2100 | ≫2100 | ≫2100 | ≫2100 | |||
| Total | 151 | 1510μg | 1540μg | ||||
No statistical significance between the total mean values of PD20 (1510) and PD40 (1540); p>0.85.
The recorded FEV1 value represents the entire bronchial tree. Continuous bronchial branching gives rise to increasingly smaller bronchi, known as small airways between (divisions 7 to 19), with an internal diameter of between 0.5 and 2mm. Forced mid-expiratory flow or FEF25–75 informs of the functional condition of this bronchial zone. While the value of FEV1 on the spirometric curve expresses the degree of bronchial obstruction with considerable reliability, FEF25–75 is more variable. Nevertheless, the value of this latter parameter is sometimes used when FEV1 is found to be within normal limits, even when calculated in relation to vital capacity (VC), that is, FEV1/FVC.9,10 Since the inflammatory and remodelling processes appear to have a greater impact upon the small airways,11–13 it is currently debatable as to whether FEF25–75 is more precise in assessing bronchial hyperresponsiveness (BHR) when the bronchoconstricting test is performed with methacholine or histamine. According to Hargreave et al.,14 in these tests it is difficult to establish the reduction cut-off points of both parameters differentiating asthmatic patients from non-asthmatic individuals – which according to these authors have been conventionally defined as 20% for FEV1 and 40% for FEF25–75 – since these limits are not usually exceeded among non-asthmatics. Objective assessment of the test may depend on the methacholine dose administered,8,15 which varies according to the different protocols presently in use. The single dose test used in our study affords a more reliable estimation of the methacholine inhaled.6
Different authors consider that in simple spirometry, FEF25–75 is highly variable and unstable, and should not be considered for the diagnosis of asthma.8,16 Regarding its usefulness in the methacholine test, Khalid et al.17 likewise consider that this parameter lacks clinical significance, as deduced from their study of 77 adult patients – 15 of whom were diagnosed with asthma, while seven had an uncertain diagnosis, and the remaining 55 were non-asthmatics. However, the authors admitted certain doubts in relation to their study, such as its retrospective design; the fact that an uncertain classification system was used; the possibility that two patients with cough as the sole symptom might have had eosinophilic bronchitis; and the possible influence of the variability of bronchial inflammation when the study was made.
In contrast, other authors consider that FEF25–75 may have appreciable value. Cirillo et al.2 performed the methacholine test in a total of 726 patients (mean age 24.7 years) diagnosed with asthma, rhinitis or rhinitis/asthma, and found an evident difference between FEV1 and FEF25–75 (>20 or a ratio of >1.24) – thus leading some sources to attribute greater value to the latter parameter in establishing the intensity of BHR in both asthmatics and in patients only diagnosed with allergic rhinitis. In another retrospective study involving 532 children between 4 and 18 years of age with suspected asthma, Drewek et al.3 concluded that although in 329 patients PD20 was not reached with the usual maximum dose of methacholine (≤16mg/ml), the decrease in FEF25–75 (>10%) proved significant from the second dose of the drug (0.25mg/ml). These authors concluded that FEF25–75 may be a useful marker for evaluation of the methacholine test, together with FEV1. In another study involving 437 children between 4 and 14 years of age diagnosed with asthma, Simon et al.4 assessed the usefulness of FEF25–75 in relation to FEV1 and also to FEV1/FVC, with the purpose of determining whether FEF25–75 offers additional information in relation to the variability of bronchial inflammation. The authors used provocation with methacholine and the bronchodilatory test with salbutamol, and concluded that in asthmatic children with normal FEV1/FVC values, FEF25–75 should be regarded as a useful parameter for evaluating respiratory function. Similar conclusions were drawn by Parker et al.18 in assessing FEF25–75 in relation to vital capacity.
Our study shows greater variability in the response to methacholine for FEF25–75 than for FEV1, coinciding with the findings of other studies.8,9 This may suggest that there is greater smooth muscle contractility in the small bronchi, or that such variability may be related to the variations in inflammation in different parts of this region, considering the extensive bronchial ramification characterising this zone.19,20 From the theoretical perspective, there should be a correlation between the methacholine doses needed to obtain the two parameters, as has been seen in some patients. However, important variability was found, since in a considerable number of cases the doses were very discordant and even in some cases the dose calculated for PD40 was higher than that required to reach PD20 (data not shown). Nevertheless, the difference in the mean values between the two parameters was not significant (p>0.85) (Table 3).
It is well known that in many patients, allergic rhinitis represents the first atopic manifestation, preceding the symptoms of asthma, which may manifest late provided the genetic predisposition is not too intense. However, these are patients in whom BHR can be demonstrated quite some time in advance,21–24 thus making it necessary to afford adequate and early management of the rhinitis. The latter in turn can be associated to tracheobronchial symptoms, since the respiratory mucosa where the allergic reaction takes place possesses similar characteristics throughout its territory.25 Several of the patients included in group 3 of our study suffered rhinitis, which in some cases was associated to symptoms of tracheobronchitis, without bronchoconstriction episodes – thus explaining why BHR was not demonstrated. Other patients in this same group, some likewise presenting rhinitis, were diagnosed with mild asthma – this correlating to the low intensity of response to methacholine. Comparison of these two series of individuals revealed no significant differences referred to the decrease in FEV1 (p>0.09), although significance was indeed found referred to the reduction in FEF25–75 (p<0.001); as a result, the latter parameter, in agreement with Drewek et al.,3 who conclude that “bronchoreactivity would be diagnosed based on the FEF25–75 response”, could have a certain predictive value.
In conclusion, in agreement with the established14 our study recognises the usefulness of PD20 to value the intensity of BHR. In contrast, PD40 showed some variability, and was not always comparable to PD20. Nevertheless, it may be useful when the dominant symptoms correspond to rhinitis or tracheobronchial conditions without dyspnoea and PD20 proves scantly demonstrative. In addition, PD40 could be of predictive value in reference to asthma in patients with few antecedents of atopy, when exposure to aeroallergens or environmental pollutants proves excessive.
Conflict of interestThe authors have no conflict of interest to declare.