Common variable immunodeficiency (CVID) is a primary antibody deficiency characterised by decreased antibody production and low or normal B-cell numbers. To elucidate the clinical and immunological heterogeneity of CVID, we studied 16 patients diagnosed with CVID.
MethodsWe analysed B, T and NK cell populations. We also assessed CD27 expression to define B-cell subsets and examined the expression of molecules important in B-cell proliferation and differentiation, such as the transmembrane activator and CALM interactor (TACI), inducible costimulator (ICOS), CD154 and CD40.
ResultsWe observed reduced B and T-cell numbers in CVID patients; this reduction was more pronounced in adults. While one group of patients (group I) showed a significant reduction in CD27+ memory B-cells, another group (group II) of patients exhibited numbers of CD27+ memory B-cells similar to the healthy donor. The frequency of B-cells and T-cells expressing CD40 and ICOS, respectively, was significantly lower in all CVID patients compared with healthy donors. Finally, a correlation between the frequency of CD27+ memory B-cells and clinical features was observed in CVID patients.
ConclusionThese results suggest that in some patients, the combined defects in both T and B-cells may account for CVID. Additionally, patients in group I exhibited an increased frequency of pneumonia and chronic diarrhoea.
After selective IgA deficiency, common variable immunodeficiency (CVID) is the most common antibody-based primary immunodeficiency. CVID patients have decreased levels of IgG and IgA and, in some cases, IgM. Patients suffer from recurrent infections, predominantly of the respiratory and gastrointestinal tracts. CVID is also associated with autoimmune, inflammatory and lymphoproliferative manifestations and malignancies.1,2 The number of peripheral B-cells are normal in most cases.3 Several cellular defects have been observed in patients with CVID, including alterations in the proliferation and activation of T-cells, irregular cytokine production and decreased expression of costimulatory molecules such as CD40L.4 However, the most noticeable defects occur in B-cells, particularly during B-cell differentiation affecting the processes of somatic hypermutation and isotype switching.5 Additionally, a small number of CVID patients have mutations in CD19, TNFRSF13B (TACI), TNFRSF13C (BAFF-R), inducible costimulator (ICOS), CD20, CD81, CD21 and MSH5.6–13
Several laboratories have proposed the classification of CVID patients based on memory B-cell numbers. Further, enumeration of memory B-cells in CVID has been proposed as a prognostic marker for respiratory disease, autoimmunity and granulomatous disease.14,15
To date, no studies of CVID patients in Mexico have been performed. For this reason, we characterised multiple immunological parameters in 16 patients diagnosed with CVID. Lymphocyte populations and the expression of molecules important for B-cell proliferation and differentiation, such as TACI, ICOS, CD154 and CD40 were analysed. Additionally, the presence of memory B-cells was assessed using CD27 expression as a marker. Thus, the main purpose of this work was to identify potential abnormalities in the expression of these molecules and to correlate any documented abnormalities with clinical features with the aim to better characterise CVID and contribute to the understanding of this pathology.
Material and methodsPatients and controlsIn total, 16 CVID patients from the Instituto Nacional de Pediatría and Centro Médico Nacional “La Raza” Instituto Mexicano del Seguro Social, Mexico City, were included in the study. All patients fulfilled the criteria for CVID based on definitions from the European Society for Immunodeficiency (ESID) and the Pan-American Group for Immunodeficiency (PAGID); these definitions stipulated a marked decreased of IgG at least two standard deviations (SDs) below the age-matched mean, reduced serum IgA and/or IgM, age of onset greater than two years and exclusion of other causes of hypogammaglobulinaemia.16
In this work, all participating patients displayed reduced levels of at least two Ig isotypes and IgG serum levels below 600mg/dL. Clinical information was compiled from each subject from medical files at the time of the study, all these manifestations are included in Table 1. This study was performed with the consent of all patients and the corresponding institutions and the study received approval from the ethics committee from each participating institution. We also included 64 controls aged 5–50 years (mean, 19 years) for phenotypic analysis. The control group comprised of 24 males and 40 females.
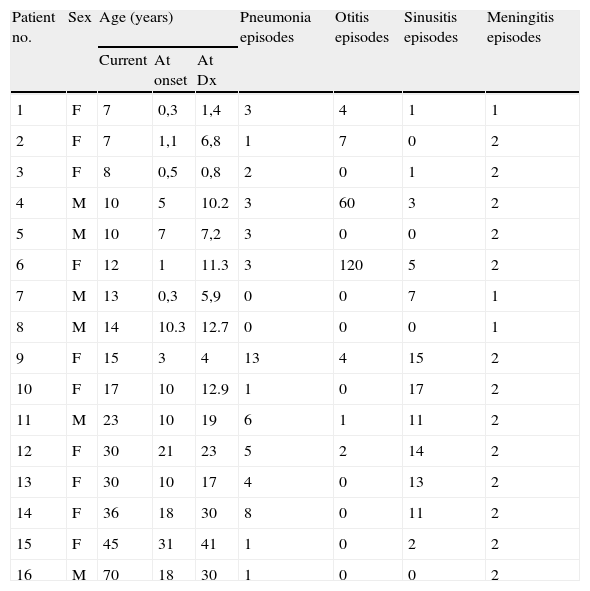
Clinical and phenotypic characteristics of CVID patients.
| Patient no. | Sex | Age (years) | Pneumonia episodes | Otitis episodes | Sinusitis episodes | Meningitis episodes | ||
| Current | At onset | At Dx | ||||||
| 1 | F | 7 | 0,3 | 1,4 | 3 | 4 | 1 | 1 |
| 2 | F | 7 | 1,1 | 6,8 | 1 | 7 | 0 | 2 |
| 3 | F | 8 | 0,5 | 0,8 | 2 | 0 | 1 | 2 |
| 4 | M | 10 | 5 | 10.2 | 3 | 60 | 3 | 2 |
| 5 | M | 10 | 7 | 7,2 | 3 | 0 | 0 | 2 |
| 6 | F | 12 | 1 | 11.3 | 3 | 120 | 5 | 2 |
| 7 | M | 13 | 0,3 | 5,9 | 0 | 0 | 7 | 1 |
| 8 | M | 14 | 10.3 | 12.7 | 0 | 0 | 0 | 1 |
| 9 | F | 15 | 3 | 4 | 13 | 4 | 15 | 2 |
| 10 | F | 17 | 10 | 12.9 | 1 | 0 | 17 | 2 |
| 11 | M | 23 | 10 | 19 | 6 | 1 | 11 | 2 |
| 12 | F | 30 | 21 | 23 | 5 | 2 | 14 | 2 |
| 13 | F | 30 | 10 | 17 | 4 | 0 | 13 | 2 |
| 14 | F | 36 | 18 | 30 | 8 | 0 | 11 | 2 |
| 15 | F | 45 | 31 | 41 | 1 | 0 | 2 | 2 |
| 16 | M | 70 | 18 | 30 | 1 | 0 | 0 | 2 |
| Gastroenteritis episodes | Allergic diseases | Bronchiectasis | Autoinmune diseases | Chronic diarrhoea | Splenomegaly | Chronic nonmalignat lymphoproliferation | Lymphadenopathy | Hepatomegaly |
| 4 | No | No | No | Yes | No | No | Yes | No |
| 3 | No | Yes | No | No | No | No | No | No |
| 1 | Yes | No | No | Yes | No | No | No | No |
| 0 | No | Yes | No | No | No | No | Yes | No |
| 0 | Yes | No | No | No | No | No | Yes | No |
| 12 | Yes | Yes | No | Yes | No | No | No | No |
| 2 | Yes | No | No | No | No | No | No | No |
| 0 | No | No | No | No | No | No | No | No |
| 1 | No | Yes | No | No | No | No | No | No |
| 6 | No | Yes | No | Yes | No | No | Yes | No |
| 6 | Yes | Yes | No | Yes | No | No | Yes | No |
| 2 | No | Yes | Yes | Yes | Yes | No | Yes | Yes |
| 9 | No | No | No | Yes | No | No | No | No |
| 4 | No | Yes | No | Yes | No | Yes | No | No |
| 2 | Yes | Yes | No | Yes | No | Yes | No | No |
| 0 | No | No | Yes | No | Yes | Yes | Yes | Yes |
To assess the different lymphocyte subpopulations, we performed flow cytometric analysis in controls and CVID patients prior to intravenous gamma-globulin (IVIG) infusion. Lymphocyte populations were enumerated in whole-blood samples and stained with the following mixtures of monoclonal antibodies (mAb): anti-CD45−FITC/anti-CD14−PE, anti-CD3−FITC/anti-CD19−PE/anti-CD45−PerCP, anti-CD4−FITC/anti-CD8−PE/anti-CD3−PerCP, anti-CD3−FITC/anti-CD16+56-PE/anti-CD45−PerCP, and, as isotype control, γ1-FITC/γ2-PE/anti-CD45−PerCP. All antibodies were purchased from BD Biosciences, San Diego, CA, USA. Samples were incubated for 20min at room temperature in the dark. After incubation, erythrocytes were lysed by suspending the cells in 500μL of FACS lysing solution (BD) for 10min. Cells were then washed with PBA (1% bovine serum albumin in PBS) and fixed using 1% formalin in PBS. To identify memory B-cell populations, peripheral mononuclear cells (PMBCs) were isolated using density gradient centrifugation with Histopaque®-1077 (Sigma-Aldrich, St Louis, MO, USA). Isolated PBMCs were then stained with a mixture of anti-CD19−APC (BD) and anti-CD27−PE (BD) antibodies; samples were incubated for 20min at room temperature in the dark. Next, the samples were washed using PBA and fixed in PBS containing 1% formalin.
Determination of CD154, ICOS, CD40 and TACI expressionCD154 and ICOS expression were assessed in activated T-cells. For CD154, PBMCs were cultured at 37°C in a 5% CO2 environment in the presence of 10ng/mL phorbol 12-myristrate 13-acetate (PMA) (Sigma-Aldrich, St. Louis Missouri, USA) and 1μg/mL of ionomycin (Sigma-Aldrich) for 12h at a density of 2×106cells/mL in RPMI 1640 medium (GIBCO-BRL, Gaithersburg, MO, USA) supplemented with 10% foetal calf serum (PAA, Morningside, QLD, AU), 1mM l-glutamine, 100units/mL penicillin and 10μg/mL streptomycin (GIBCO). After activation, PBMCs were stained with a mixture of anti-CD3−PerCP (BD), anti-CD154−PE (BD) and anti-CD69−FITC (BD). To examine ICOS expression, PBMC activation was performed overnight using the conditions described above. Cells were then stained with a mixture of anti-CD3−PerCP (BD), anti-ICOS-PE (BD) and anti-CD69−FITC (BD) following the protocol described above. Non-activated cells were used as negative controls for both CD154 and ICOS expression. CD69 expression was used as a positive control for T-cell activation. CD40 and TACI expression in B-cells were determined by staining PBMCs with a mixture of anti-CD40−PE (BD) or anti-TACI-PE (BD) and anti-CD19−APC (BD). Samples were acquired using a FACSCalibur® flow cytometer (BD); data analysis was performed using FlowJo 7,2,4 software (Tree Star. Inc., Ashland, OR, USA).
Statistical analysisDescriptive data are presented as the mean±standard deviation. We compared continuous variables between the age-matched groups using the Mann–Whitney U-test and nominal variables using Fisher's exact test. Differences between groups were considered significant when p<0,05. Data were analysed using GraphPad Prism version 4,00 for Windows (GraphPad Software, San Diego, CA, USA, www.graphpad.com).
ResultsPatient population and clinical phenotypeOf the 16 CVID patients assessed, ten (63%) were female. There was one pair of siblings with CVID, and one patient had a relative with selective IgA deficiency; the demographic and clinical characteristics of the patients are summarised in Table 1. While the mean age of disease onset was 12.2 years, the mean age of diagnosis was 14.6 years. In CVID patents, the mean time from disease onset to diagnosis was 5,4 years (DS 4,2 years). Frequently observed clinical manifestations included pneumonia (88%, n=14), sinusitis (75%, n=12), chronic diarrhoea (75%, n=12), bronchiectasis (56%, n=9) and lymphadenopathy (37%, n=6).
The immunological features of CVID patients are shown in Table 2, the median values of serum IgG, IgM and IgA at diagnosis were 286mg/dL, 42mg/dL and 18mg/dL, respectively. The main feature that distinguished CVID patients was a reduced CD4/CD8 ratio (50%); however, two CVID patients (12.5%) had an exceptionally high CD4/CD8 ratio.
Immunological features of CVID patients.
| Patient no. | Age (years) | Sex | IgG (mg/dl) | IgM (mg/dl) | IgA (mg/dl) | Total leucocytes (mm3) | Total lymphocytes (mm3) | T lymphocytes (CD3+) (mm3) | CD4+ lymphocytes (mm3) | CD8+ lymphocytes (mm3) | B lymphocytes (mm3) |
| 1 | 7 | F | 331 | 31 | 8 | 8400 | 1260 | 819 | 524 | 199 | 258 |
| 2 | 7 | F | 133 | <17 | <22 | 5500 | 2321 | 1880 | 940 | 865 | 162 |
| 3 | 8 | F | 495 | 49 | 13 | 5000 | 1550 | 1070 | 385 | 446 | 186 |
| 4 | 10 | M | 418 | 187 | <25 | 7300 | 2131 | 1322 | 291 | 986 | 83 |
| 5 | 10 | M | 517 | 95 | 39 | 7100 | 2982 | 2415 | 792 | 1505 | 189 |
| 6 | 12 | F | 206 | <16 | <22 | 8900 | 4512 | 3925 | 2041 | 1727 | 315 |
| 7 | 13 | M | 476 | 51 | <2 | 9000 | 4230 | 3185 | 2777 | 86 | 478 |
| 8 | 14 | M | 295 | 21 | <25 | 4800 | 1008 | 689 | 420 | 193 | 174 |
| 9 | 15 | F | 293 | 16 | 4 | 8500 | 2919 | 2335 | 574 | 1642 | 44 |
| 10 | 17 | F | 261 | <5 | <6 | 11.200 | 1456 | 1267 | 532 | 634 | 96 |
| 11 | 23 | M | 154 | <17 | <25 | 4000 | 452 | 362 | 257 | 90 | 42 |
| 12 | 30 | F | 293 | 69 | <25 | 14.400 | 2448 | 2154 | 1702 | 164 | 12 |
| 13 | 30 | F | 261 | <17 | <25 | 4600 | 566 | 526 | 131 | 381 | 18 |
| 14 | 36 | F | 344 | <17 | <23 | 4000 | 1240 | 992 | 516 | 397 | 128 |
| 15 | 45 | F | 43 | 19 | 7 | 3000 | 303 | 274 | 121 | 135 | 2 |
| 16 | 70 | M | 60 | 42 | 13 | 2400 | 408 | 363 | 124 | 210 | 20 |
| NK lymphocytes (mm3) | Monocytes mm3 | Ratio CD4/CD8 rv1 0,9–1,9 | CD19+CD27+ (%) | CD19+CD27+ (mm3) | CD19+CD27− (%) | CD19+CD27− (mm3) | CD40/CD19 (%) | ICOS activated (%) | CD154 activated (%) | TACI (%) |
| 135 | 109 | 2,6 | 8 | 21 | 82 | 212 | 85 | 44 | 96 | 84 |
| 369 | 149 | 1,1 | 48 | 78 | 52 | 84 | 88 | 10 | 69 | 48 |
| 326 | 105 | 0,86 | 30 | 56 | 70 | 130 | 88 | 38 | ND | 37 |
| 821 | 774 | 0,3 | 4 | 3 | 96 | 80 | 78 | 11 | 6 | 24 |
| 924 | 192 | 0,52 | 30 | 57 | 70 | 132 | 91 | 13 | 42 | 33 |
| 225 | 551 | 1,2 | 7 | 22 | 93 | 294 | 73 | 83 | 82 | 21 |
| 469 | 603 | 32.3 | 42 | 201 | 58 | 277 | 88 | 74 | 65 | 25 |
| 53 | 250 | 2,2 | 38 | 66 | 62 | 108 | 67 | 30 | 12 | 48 |
| 613 | 287 | 0,35 | 36 | 16 | 64 | 28 | 85 | 44 | 96 | 84 |
| 210 | 448 | 0,84 | 27 | 26 | 73 | 70 | 92 | 17 | 90 | 47 |
| 113 | 320 | 2,8 | 6 | 3 | 94 | 39 | 95 | 32 | 10 | 64 |
| 362 | 864 | 10.4 | 23 | 3 | 77 | 9 | 90 | 16 | 16 | 27 |
| 29 | 147 | 0,34 | 12 | 2 | 88 | 16 | 64 | 41 | 13 | 23 |
| 220 | 520 | 1,3 | 12 | 15 | 88 | 113 | 73 | 16 | 12 | 14 |
| 62 | 195 | 0,89 | ND | ND | ND | ND | ND | 16 | 96 | ND |
| 39 | 48 | 0,59 | 31 | 6 | 69 | 14 | 99 | 17 | 16 | 40 |
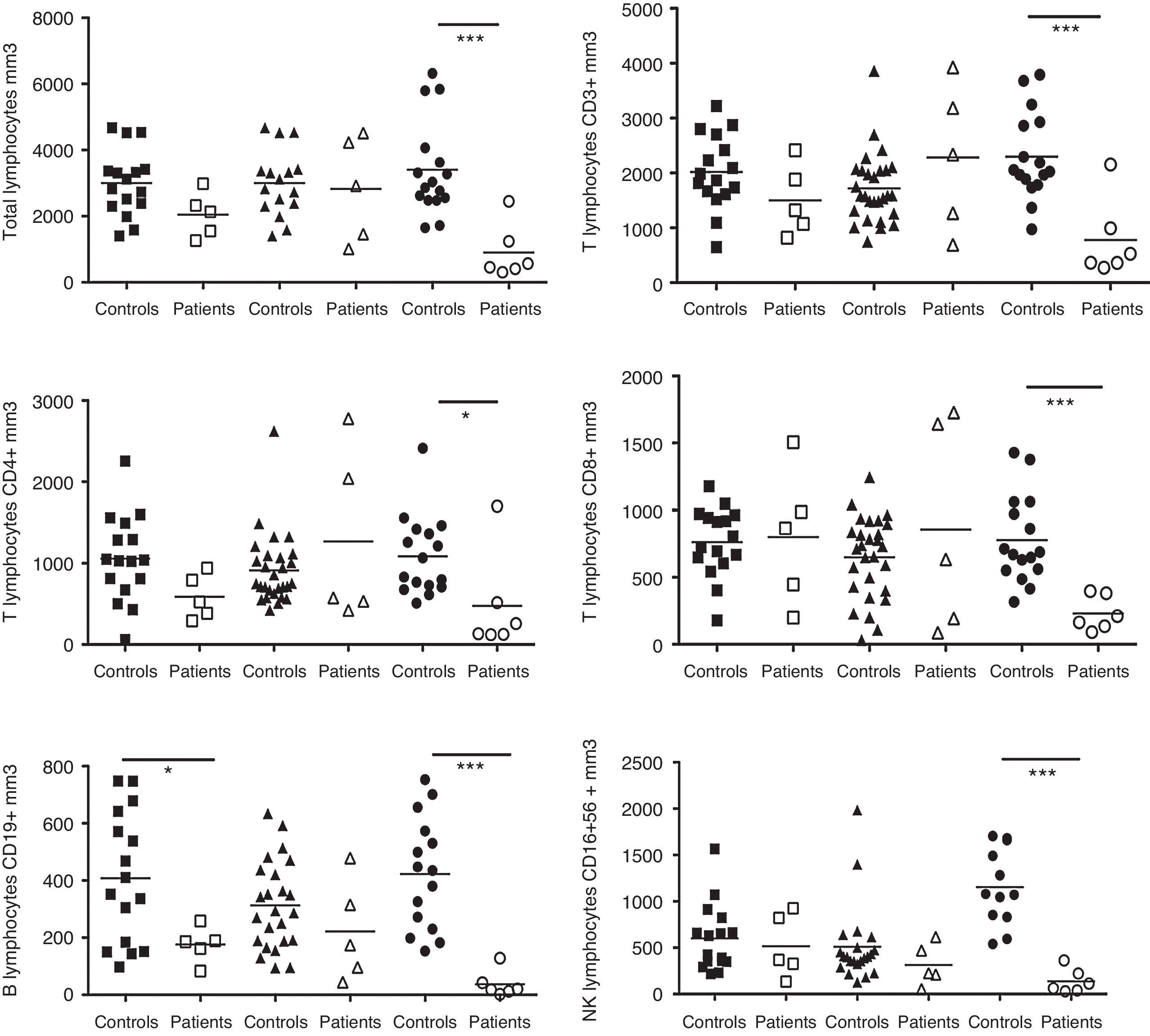
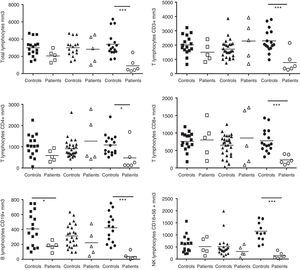
The absolute numbers of B, CD4, CD8, total T and NK cells from the patients were analysed and compared with the controls. The patients were divided into the following three groups based on age: children, 5–10 years old (n=5); teenagers, 11–17 years old (n=5); and adults, 17 years old and older (n=6). These groups were compared with the following age-matched control groups: children (n=17), teenagers (n=28) and adults (n=19) (Fig. 1). Adult CVID patients exhibited a pronounced reduction in all lymphocyte populations compared with HD, including CD3 cells (p=0.0006), CD4 T-cells (p=0.0245), CD8 T-cells (p=0.0007), CD19 B-cells (p=0.0002) and CD16+56 NK cells (p=0.0001). Children with CVID showed a moderate reduction in CD19 cells (p=0.0386).
Analysis of lymphocyte populations. Flow cytometric analysis of total lymphocytes, T-cells, T cell subsets, B-cells and NK cells in absolute numbers (cells/mm3) from children (□, n=5), teenagers (▵, n=5) and adults (○, n=6) with CVID; these groups were compared with control children (¿, n=17); teenagers (▴, n=28) and adults (¿, n=16). Differences between patients and controls were compared using the Mann–Whitney U-test. (*) Significant, p<0,05; (**) very significant, p<0,01; and (***) highly significant, p<0,001.
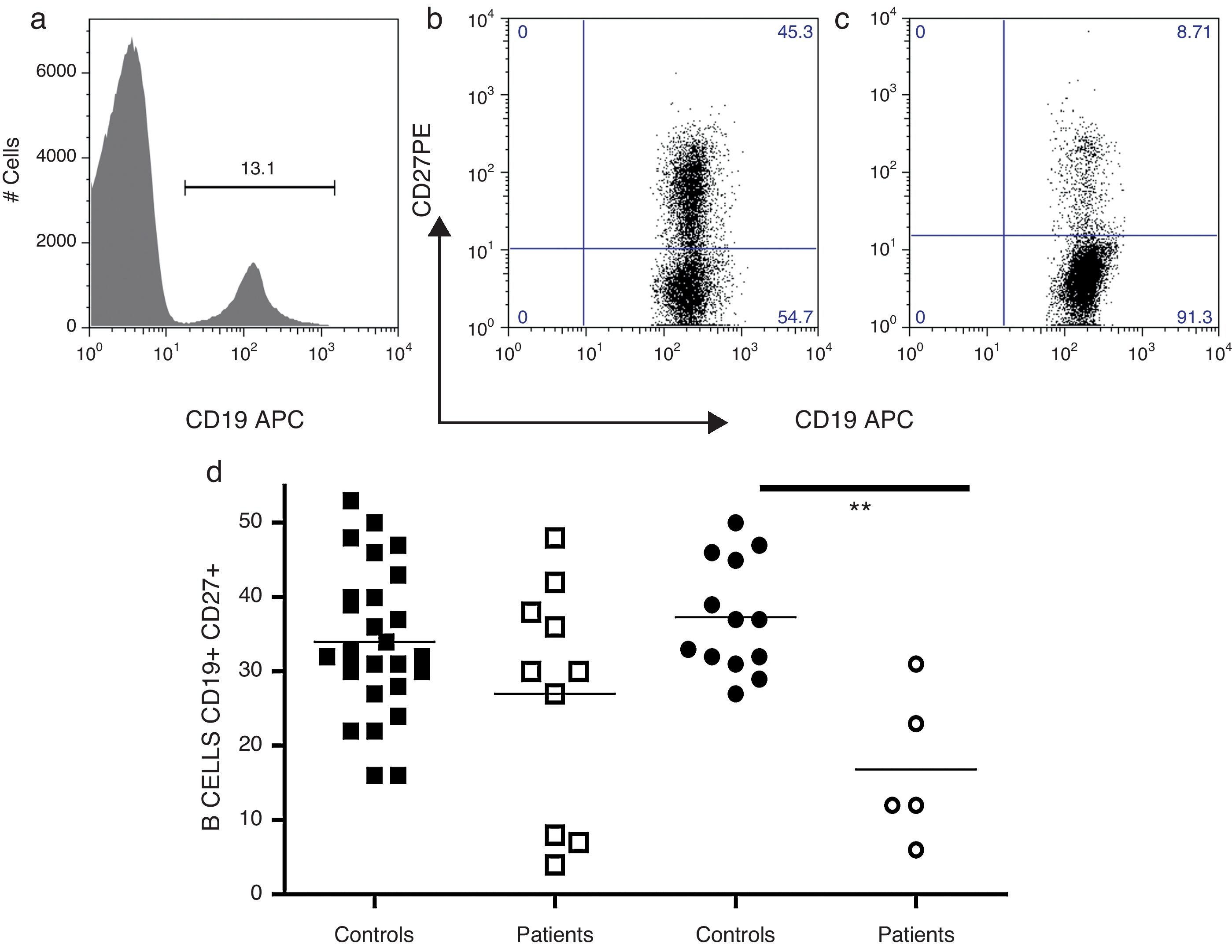
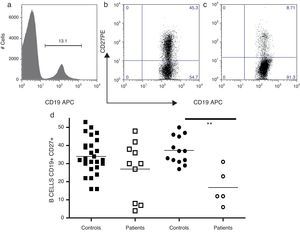
The frequency of memory B-cells in 15 CVID patients were examined; an adult patient was not examined because she had less than one percent of peripheral B-cells (Fig. 2). The patients were divided into two groups, children and teenagers (n=10) and adults (n=5), and compared with age-matched control children and teenagers (n=27) and adults (n=13). CD27 expression has been used as a surrogate marker for memory B-cells. Thus, as shown in Fig. 2a, the simultaneous evaluation of CD19 and CD27 enabled the identification of naïve (CD19+, CD27−) and memory B-cells (CD19, CD27+) in the peripheral blood. As shown in Fig. 2b, adult CVID patients showed significantly reduced levels of memory B-cells compared with age-matched controls (p=0.0036).
Analysis of B-cell subpopulations. Representative flow cytometric analysis of B-cell subsets. PMBCs were stained with mAbs against CD19 and CD27. After gating on lymphocytes according to forward (FSC) and side scatter (SSC), B-cells were identified according to CD19 expression (a). A CD19/CD27 dot plots were utilised to determine the frequency of the following B-cell subsets: total memory B-cells (CD19+CD27+) and naïve B-cells (CD19+CD27−). Controls (b) and CVID patients (c). Frequencies of total B memory cells from children and teenagers (○, n=10) and adults (□, n=5) with CVID were compared with control children and teenagers (¿, n=27) and adults (¿, n=13). Differences between patients and controls were compared using the Mann–Whitney U-test (Fig. 2d). (*) Significant, p<0,05; (**) very significant, p<0,01; and (***) highly significant, p<0,001.
CVID patients were also divided into two groups according to the frequency of memory cells. Group I comprised of patients whose memory B-cell frequencies were at least one standard deviation below the values obtained for memory B-cells from age-matched controls (mean B cells CD19+CD27+ of children and teenager controls=34%; DS=9,8 and adult controls=37%; DS=7,5). Patients in Group II had normal or elevated numbers of memory B-cells. Table 3 shows a summary of the clinical manifestations of CVID in each group. Patients with a low frequency of memory B-cells showed a significant increase in the incidence of chronic diarrhoea (p=0,041) and pneumonia (p=0,026) compared with patients with normal or elevated frequencies of memory B-cells.
Clinical manifestations of CVID patients grouped according to percentage of memory B cells.
| Clinical characteristics | Group I (n=7) | Group II (n=8) | p value |
| Mean age | 21 | 19 | 0,487 |
| Mean age at onset of symptoms | 9,3 | 6,3 | 0,684 |
| Mean age at diagnosis | 16 | 14 | 0,224 |
| Number of patients with history of pneumonia | 7 | 6 | 0,467 |
| Mean event rate of pneumonia | 5 | 4 | 0,026* |
| Number of patients with complications of bronchiectasis | 5 | 3 | 0,315 |
| Number of patients with history of otitis | 5 | 2 | 0,132 |
| Mean event rate of otitis | 37 | 6 | 0,152 |
| Number of patients with history of sinusitis | 7 | 4 | 0,077 |
| Mean event rate of sinusitis | 8 | 10 | 0,242 |
| Number of patients with history of gastroenteritis infection | 6 | 5 | 0,569 |
| Mean event rate of gastroenteritis infection | 6 | 3 | 0,061 |
| Number of patients with chronic diarrhoea | 6 | 2 | 0,041* |
| Hospitalisations | 7 | 8 | 1 |
| Mean event rate of hospitalisations | 6 | 4 | 0,101 |
| Mean event rate of days of stay | 59 | 63 | 0,955 |
*p<0,05.
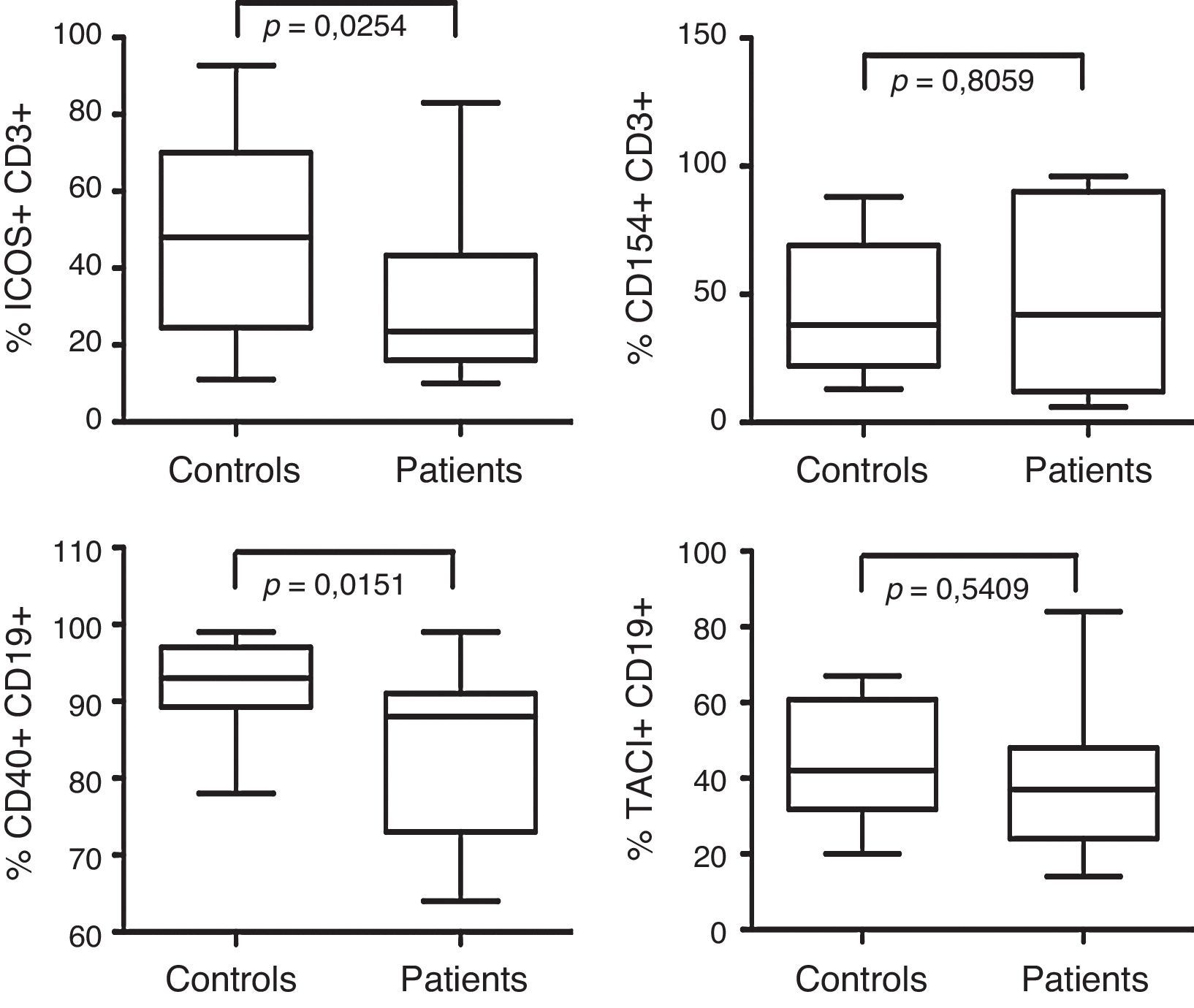
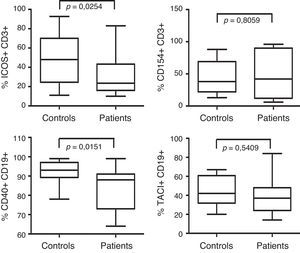
The frequency of co-stimulatory molecules expression is shown in Fig. 3. When B-cell CD40 expression was analysed, we found significant differences between 15 patients and 18 controls (p=<0.0151). No differences were observed in CD154 expression in T-cells from 16 CVID patients compared with 17 controls (p=0,8). TACI expression was also similar between 15 CVID patients and 14 controls (p=0,54). However, ICOS expression on T-cells was reduced in the 16 CVID patients analysed compared with 17 controls (p=0.0254).
Analysis of CD40, CD154, TACI and ICOS expression. ICOS and CD154 expression was examined in activated CD3+ T-cells. ICOS expression was analysed from CVID patients (n=16) and controls (n=17). CD154 expression was analysed from CVID patients (n=16) and controls (n=17). TACI expression was analysed in CD19+ B-cells from CVID patients (n=15) and controls (n=14). B-cell CD40 expression was assessed in CVID patients (n=15) and controls (n=18). Dates are presented as box plots displaying medians, 25th and 75th percentiles and 10th and 90th percentiles as vertical lines. Differences between patients and controls were determined using the Mann–Whitney U-test.
CVID is a highly heterogeneous immunodeficiency syndrome in which B cell and T-cell defects have been shown.17 In this study, eight out of 16 CVID patients showed significant alterations in CD4 and CD8 T-cell populations. Consistent with an observation by Wright,18 these patients also exhibited reduced CD4/CD8 ratios.
A recent explanation for the decrease in CD4 T-cells in CVID patients involves spontaneous apoptosis and a reduction in thymus size. Because CVID is a progressive disease, patients who experience phenotypic changes such as reduction of T and B-cells can also develop complications over time.17 Paediatric patients predominately showed a significant reduction in CD19 B-cells, whereas in adults, all lymphocyte populations were reduced. Although these differences between adult and paediatric patients have been described previously, no clear aetiopathogenesis has been established. The presence of both T-cell and B cell abnormalities suggests distinct disease mechanisms and indicates that different approaches for genetic research should be used. A molecular explanation for the differences observed in the absolute number of lymphocyte has not been described. However, it is possible that defects in several molecules may lead to flawed interactions between B and T-cells, potentially explaining the various clinical changes observed over time in CVID patients.19 Importantly, a substantial defect was noted not only in the reduction of overall T cell population, but also in NK and B-cells; consequently, some of these patients could be considered as having combined immunodeficiency with a common early defect in lymphocyte differentiation.20 Two patients with a form of adenosine deaminase deficiency have been reported to suffer from opportunistic infections in adulthood.21 Therefore, this pathway should be further investigated in CVID patients.
Classification of B cell differentiation through the characterisation of circulating B-cell subpopulations is an important tool to understand the clinical manifestation in CVID. Splenomegaly, granulomatous disease and infections have been associated with reduced levels of switched memory B-cells.14,15 A limitation of this study was the lack of measurement of switched memory B-cells. However, we used CD27 expression as surrogate marker for memory B-cells. CD27 expression increases gradually with age; thus, in adults, approximately 40% of circulating B cells are CD27+.5 However, we found that adult CVID patients had a significant reduction of memory B-cells compared with age-matched controls. This finding indirectly confirms previous results, indicating impaired germinal centre function over the progressive course of CVID.22
Among the CVID patients, we detected some with a significant reduction in the memory B-cell compartment (Fig. 2b). The patients with reduction in memory B-cells were classified in Group I. A relevant feature in these patients was the high prevalence of chronic diarrhoea and an increased number of episodes of pneumonia compared with CVID patients with normal levels of memory B-cells. Memory B-cells undergo somatic hypermutation and generate immunoglobulins rapidly and vigorously during a secondary immune response; their absence in some CVID patients may partially explain the higher prevalence of infections observed in CVID patients in Group I (Table 3).23
The clinical phenotype of CVID has been attributed to mutations in genes that encode molecules such as ICOS, TACI, CD19, BAFFR, CD21, CD20 and CD81. However, mutations in each of those genes are present in less than 20% of CVID patients.24 All patients analysed in this study exhibited normal TACI expression; however, a functional evaluation and sequencing are needed to definitively rule out a genetic defect in TNFRSF13B.
Although ICOS expression on T-cells from CVID patients was detectable, it was significantly reduced compared with controls. Reduced ICOS expression may indicate an abnormal mechanism of communication between B and T-cells and play a key role in the pathogenesis of CVID.
In contrast with a previous report, the frequency of CD40 expression in B-cells from CVID patients was significantly reduced.25 CVID patients not only had less B-cells, but B-cell CD40 surface expression was also diminished. Due to the important role of CD40 in B cell differentiation, this reduction in CD40 expression may account for some of the defects found in these patients.
In this study, we identified possible candidates for a more detailed analysis. For example, patients with low ICOS expression should be examined for mutations. Additionally, Btk should also be considered, as mutations in this enzyme have been reported to be responsible for a CVID-like disease that may represent a mild form of XLA.26–28 In fact, one patient who was initially diagnosed with CVID had a mutation in Btk and was excluded from this study. Taken together, we believe that the insights gained in this work may help to improve the diagnosis of CVID. Further, similar to recently described mutations in CD20 and CD81 (7; 11), our findings may provide us with the opportunity to find new causes for CVID.
The diverse and widespread distribution of these defects further highlights the heterogeneity present among CVID patients and indicates that multiple factors likely play a role in generating the CVID phenotype.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The research was conducted at National Institute of Pediatrics under approved protocol (#29/2009). The author for correspondence is in possession of this document.
Protection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors have no conflict of interest to declare.
This work was partially supported by the Instituto de Ciencia y Tecnología del Distrito Federal (grant ICYTDF/326/2009), the Consejo Nacional de Ciencia y Tecnología (CONACYT, México) (grant #’s 56836 and 58945); and the Fundación Mexicana para los Niñas y Niños con Inmunodeficiencias Primarias A.C.
Gabriela López-Herrera, Francisco Espinosa-Rosales, Ethel García-Latorre and Leopoldo Santos-Argumedo are SNI fellows. Ethel García-Latorre is a fellow of COFAA, EDI, and IPN. Laura Berrón-Ruiz was the recipient of a doctoral (176353) and postdoctoral (CVU 44813) scholarship from CONACYT, México.
The authors would like to thank Dr Chiharu Murata and MD/PhD Martha Guevara Cruz for their help with the statistical analysis and Héctor Romero-Ramírez, Víctor Hugo Rosales-García; and Carlos Eduardo Gomez Gaitán for their technical assistance.