The aim of this study was to assess the sensitivity profile of the population of Fez and Casablanca in Morocco to dry broad bean (Vicia faba), and to investigate the effect of food processing (heat and/or enzymatic hydrolysis by pepsin) on the human IgE binding capacity to broad bean proteins (BBP).
MethodsSera samples from 146 patients with atopic hypersensitivity were recruited in order to evaluate specific IgE levels to native and processed broad bean proteins by ELISA. Under the same conditions, we assessed the immunoreactivity of rabbit IgG obtained by immunisation with native BBP.
ResultsHigh IgE levels to BBP were found; in fact, 79.3% of children and 80.4% of adults had positive values. The heat treatment (70°C during 60min) of dry beans proteins showed slight reduction in recognition of these antigens by rabbit IgG (22%) and by human IgE (12%). Pepsin hydrolysis decreased rabbit-IgG recognition by 55% in the first 30min of treatment. In contrast, and under the same conditions, pepsin increased human-IgE recognition with an average of 143% for all patients. However, the combination of the two treatments (heating and pepsin digestion) showed a decrease of 16% in BBP recognition for all patients.
ConclusionsThis study demonstrates a high sensitivity of a Moroccan population to broad bean proteins which was resistant to heat and digestion by pepsin.
Food allergies are a pressing worldwide problem, and their prevalence seems to be on the rise. They can induce serious systemic symptoms that affect the quality of life. They are found in 5% of young children and 4% of adults in Western countries.1
Food legumes play an important role in the human diet, providing a high proportion of proteins. Their consumption is very frequent in the Mediterranean area. Broad beans (Vicia faba L.), which belong to the Fabaceae family, are protein-rich legume seeds that are typically used for animal feed and human food. Broad beans are among the most consumed food in the Moroccan diet. Many case reports have indicated that legumes are responsible for allergic reactions. In Spain, lentils and chickpeas are the most frequent cause of allergic reactions to legumes in children.2,3 In India, allergies to kidney beans, chickpeas and peanuts are common.4 Peanut and soy are the legumes most frequently involved in human food allergies in countries such as the United States, the United Kingdom and Japan.5,6 In Morocco, peanuts are identified as an important food causing allergies.7,8 Allergic reactions to broad beans have rarely been reported in the literature, but a few cases have been observed previously in Italy9,10 and Spain.11
In order to manage and to minimise the risk of food allergies, investigations have studied the effect of food processing on food allergenicity. In legumes, it has been shown that heating or enzymatic hydrolysis processing may enhance, reduce, or eliminate their allergenic potential.12–17
The objective of this study was to evaluate the sensitivity profile of the Moroccan population from the two cities of Fez and Casablanca to broad bean proteins, and to investigate the modulation of this sensitivity by heat and/or enzymatic hydrolysis.
MethodsPatientsThe work was conducted on a sera-bank, composed of samples obtained from 155 volunteers. From these patients, nine were non-atopic, and 146 atopic. The atopic-patients were consulted for hypersensitivity, addressed by dermatology and pneumology services to laboratories to measure their total IgE. The patients were recruited from July 2010 to December 2010 after ethical approbation.
After formal consent from each patient, a serum sample was collected at the University Hospital Centre of Fez as well as from biomedical laboratories in Fez and Casablanca. The collected sera were stored at −20°C until used. These patients had not been sensitised to dry broad bean, nor were they challenged orally.
Extraction and treatment of the broad bean proteinsBean seeds were very finely ground. The powder or flour obtained was defatted with chloroform and then dried before proteins were extracted by suspending the samples in PBS (phosphate buffer solution pH 7.4) at 20% (w/v). The mixture was stirred for 2h, filtered and then centrifuged at 3000rpm for 15min at 4°C. The collected supernatant, considered as native broad beans proteins (BBP), was frozen at −20°C until use.
The native BBP was then treated in four different ways. It was either (1) heated at different temperatures (70, 80, 90 and 100°C) for 60, 120 and 180min; (2) treated in an acidic (pH 2) or basic (pH 11) medium for 60, 120, 180, and 210min at 37°C; (3) digested by pepsin (hog stomach, 3354U/mg) at a concentration of 50U/ml in an acidic environment (pH 2) during 30–210min at 37°C; or (4) processed by a combination of the two treatments (heating and enzymatic digestion; 1 and 3).
Production of polyclonal antibodies against the BBPTo study the immunoreactivity of antibodies with BBP, we prepared IgG antibodies against native BBP. These antibodies were obtained after immunisation of rabbits against the native BBP using Freund's adjuvant.
The BBP were injected subcutaneously at several points on the animals’ back in combination with complete Freund's adjuvant for the first injection and with incomplete Freund's adjuvant in subsequent immunisations at one week intervals. After one month, animals were sacrificed and blood samples were collected in dry tubes. After centrifugation for 15min at 3000rpm at 4°C, the sera were supplemented with sodium azide 0.02% and frozen at −20°C.
IgE determinationsTotal IgE was evaluated by direct ELISA as described before.18 Briefly, diluted human sera were placed in 96 micro-titration plate wells and incubated overnight at 4°C. The non-specific sites were saturated with bovine serum albumin (BSA) 0.25% (200μl/well). Then, 100μl of human anti-IgE peroxidase conjugate was added and immune complex revealed after the addition of 0.05% of orthophenylenediamine (OPD). Absorbance was measured at 490nm by an ELISA reader (LabsystemsMultiskan MS). Positive and negative controls were included in each plate to check the specificity and sensitivity of each measure.
For specific IgE, the BBP diluted at 0.5mg/ml in PBS was deposited on a micro-titration plate (100μl/well) indirect ELISA was used. After overnight incubation at 4°C, wells were washed, saturated with bovine serum albumin 0.25% and the plate was treated the same way as for the total IgE determination.
The binding of rabbit IgG to BBP was determined by ELISA similarly to that of specific IgE.
Polyacrylamide gel electrophoresis of BBPBeans proteins were separated by 12% (w/v) polyacrylamide gel electrophoresis under denaturing or non-denaturing conditions. The native and treated proteins were denatured by boiling for 3min in the presence of SDS 10% and β-mercaptoethanol 0.8% (denaturing conditions). The migration was done under a 25mA current and the gel was stained with 0.1% Coomassie blue R250.
EthicsThis study was approved by the ethics committee at The University Hospital Centre of Fez.
All experiments using animals have been conducted according to national and international laws.
Statistical analysisStatistics analysis was based on the Student's t-test taking P<0.05 as the limit of significant value.
ResultsSample descriptionThe total number of patients recruited for this study was nine non-atopic and 146 atopic. Non-atopic patients were adults whose age varied from 26 to 80 years composed of four men and five women. Atopic patients were composed of 98 children (67%) and 48 adults (33%). The paediatric population ranged from 1 to 15 years, with a mean of 7.5 years. Adults were composed 73% of women (17–57 years with a mean of 35 years) and 27% of men (19–55 years with a mean of 37 years).
Distribution of IgE levelsAll patients (atopic and non-atopic) recruited had been tested for total IgE which varied between 2 and 80IU/ml. Results showed a mean value for children of 13.3IU/ml. and for adults of 26IU/ml. Among 155 patients, 153 had been tested for specific IgE representing 99% of all children and 96% of all adults recruited.
For non-atopic persons, only one adult (women, 33 years) from the nine tested; showed a positive value for specific IgE of 3IU/ml to BBP.
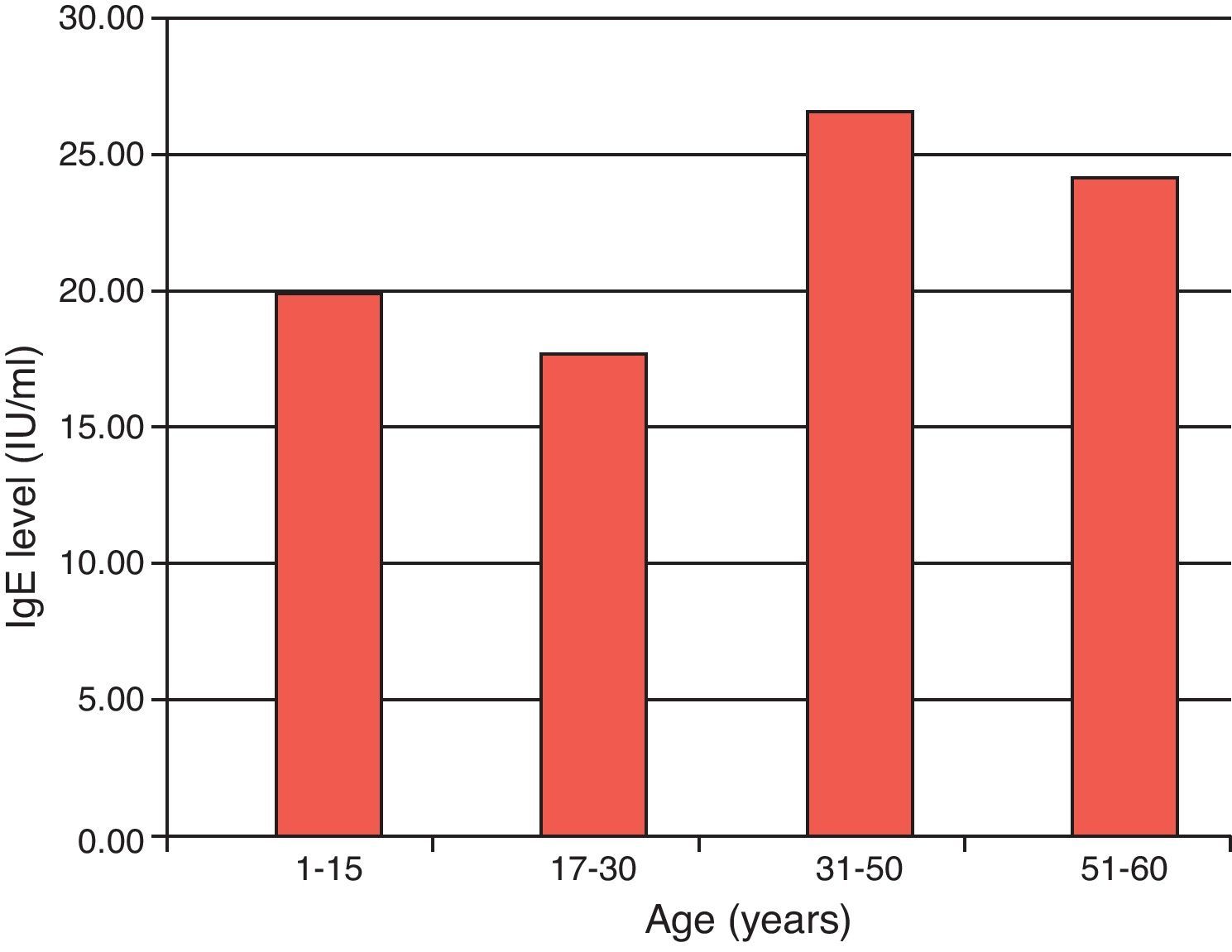
The distribution of specific IgE in the atopic population showed low values for the age below 30 years and high values were observed for more aged population (Fig. 3). From this population, 97 children tested showed that 79.3% had positive values (>2IU/ml) for specific IgE with a mean value of 25.2IU/ml, ranging from 2 to 69.6IU/ml among which 44.3% had levels higher than 20IU/ml. For atopic adults, the mean of specific IgE values was 27.7IU/ml ranging from 2 to 69.4IU/ml. Thirty-seven of them (80.4%) had positive levels and are composed of 29.7% (11) men and 70% (26) women. 54% of these adults indicated levels higher than 20IU/ml where 19% were men and 35% women.
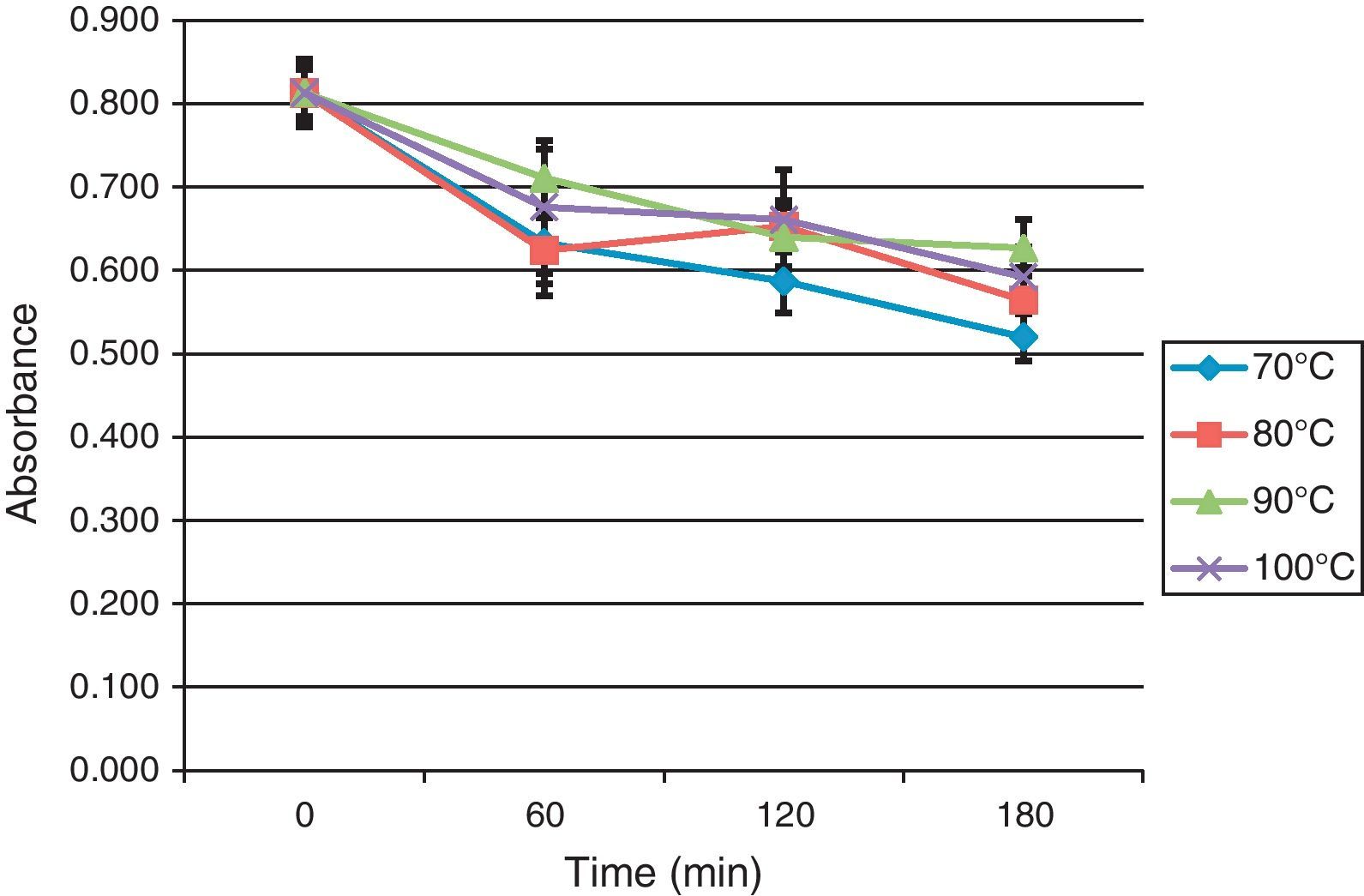
Effect of heating, pH, and enzymatic digestion on the recognition of BBP by rabbit IgGWe have studied the effect of temperature, pH, and enzymatic digestion on the immunoreactivity of PBB with rabbit IgG prepared against native BBP. BBP were heated at 70°C, 80°C, 90°C and 100°C at different time intervals varying from 30min to 180min. Treated samples were then used for IgG binding evaluation using ELISA. Regarding BBP heat treatment (Fig. 1), there was a decrease of IgG binding to BBP at all temperatures studied after the first hour of treatment with a maximum reduction of 23% at 70°C, which reached 36% after 180mn.
Effect of heat treatment of BBP on their recognition by rabbit IgG.
The effect of temperature was studied in N=3 experiments using rabbit IgG anti-native BBP. The binding of IgG was evaluated by ELISA to determine the response of IgG binding to BBP heated at different temperatures over time.
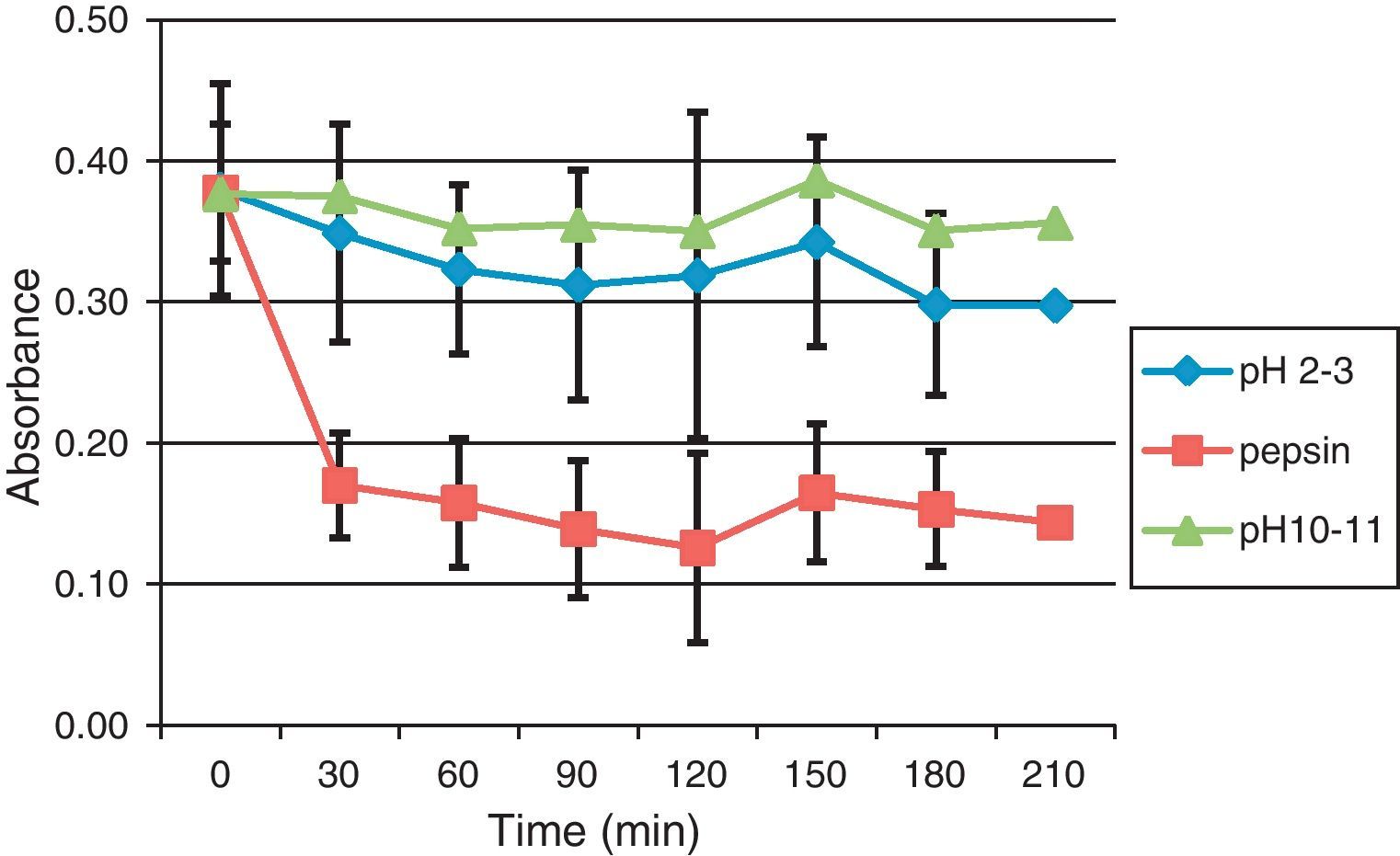
For pH treatment, BBP were incubated at 37°C in an acidic solution (pH 2) or in an alkaline solution (pH 11). Thereafter, BBP were used for ELISA to evaluate their recognition by rabbit IgG. The results of the treatment with pH11 showed (Fig. 2) that the binding of rabbit IgG to BBP remains relatively stable during all treatment when compared to initial values. The acidic pH slightly decreased the binding of IgG to BBP by 18% during 90min and by 22% during 180min to 210min.
Effect of BBP treatment under acid, alkali and enzymatic hydrolysis on their recognition by rabbit IgG.
The effect of these treatments was studied in N=3 experiments using rabbit IgG anti-native BBP. The binding of IgG was evaluated by ELISA with determining the response of IgG binding to BBP hydrolysed in alkali, acid or by pepsin at 37°C over time.
On the other hand, pepsin hydrolysis in an acidic environment greatly impaired the binding of IgG to BBP which was reduced by 55% in the first 30min of treatment, and then continued to fall to 67% during 120min and remained relatively constant thereafter.
Immunoreactivity of treated BBP with human IgEIn this section, we determined the change in the recognition of treated BBP (by heating, pH, and pepsin) by human IgE. To do this, we used human sera from nine patients with anti-BBP IgE levels higher than 41IU/ml. Patients were composed of six children (two boys and four girls) that ranged from 3 to 13 years of age, and three adult women. Specific IgE ranged from 41.60 to 69.64IU/ml (Table 1).
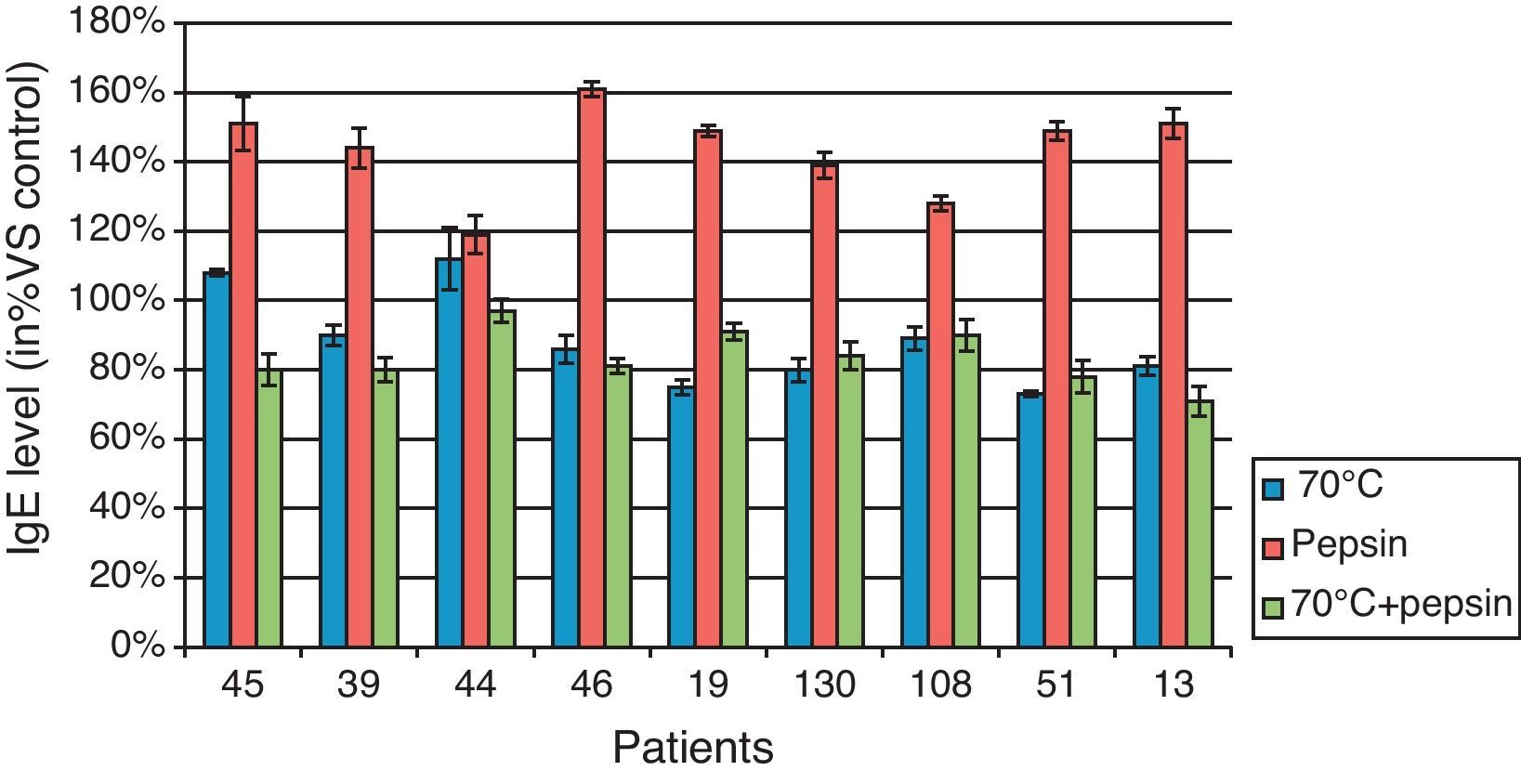
When BBP were heated at 70°C for 60min, the results reported (Fig. 4) show a slight reduction in IgE recognition for all patients ranging from 0 to 27%. Only one third of these patients showed a decrease greater than 20% in IgE recognition.
Effect of BBP heating and enzymatic hydrolysis on their recognition by human IgE.
Figure represents the IgE level in % compared to data obtained in control (No treated BBP). Values were obtained from triplicate assays for each patient in the three different conditions, heating, pepsin hydrolysis and combination of these treatments. In x-axis, numbers represent the laboratory identification number of each patient's sera.
Under pepsin hydrolysis, patients did not demonstrate any reduction in IgE recognition. In contrast, we noticed for all patients an increase in IgE recognition of 143% on average due to the heat treatment. When heating and pepsin digestion were combined, all patients showed an average reduction of 16% in the recognition of BBP, ranging from 3% to 29%, with 67% of these patients showing a decrease in IgE recognition greater than 15%.
Electrophoretic analysis (SDS-PAGE)The protein compositions of various beans extracts (native, heated and/or treated with pepsin) were compared by polyacrylamide gel electrophoresis under denaturing and non-denaturing conditions (Fig. 5). Before treatment, the native bean extracts under non-denaturing conditions showed an electrophoretic profile consisting of seven major protein bands of molecular weights ranging from 27 to 91kDa., but under denaturing conditions, we observed a disappearance of a protein band of molecular weight 57kDa. We noticed the same pattern after heat treatment under denaturing and non-denaturing conditions.
However, for the broad bean extracts treated with pepsin or “pepsin and heating” there is an important attenuation of all proteins bands under denaturing and non-denaturing conditions.
DiscussionThe present study was to evaluate food sensitivity in the Moroccan population from two regions (Fez and Casablanca) to broad bean proteins, and to assess the effect of thermal processes and/or enzymatic digestion. In fact, we established a serum bank of 146 patients with atopic hypersensitivity in order to investigate their level specific IgE directed against broad bean proteins and to compare the reactivity of IgE with treated proteins.
In the present study, 79.3% of children and 80.4% of adults had positive values of IgE to broad beans (>2IU/ml). Of these, 44.3% of children and 54% of adult samples gave values higher than 20IU/ml. The IgE levels show a very high sensitivity of the studied population to broad bean proteins which can be explained by a major production and consumption of broad beans which represent one of the main food legumes in Morocco. The average consumption of broad beans was estimated about 2.4kg per capita per year, which exceeds the consumption levels of other food legumes.19
Sensitisation to legumes is very important in some countries related to their culinary habits. In Spain, where chickpeas and lentils are a predominant cause of allergic reaction in children, sensitivity to legumes is the fifth most common cause of food allergy.2 Rare cases of allergic reaction to broad beans have been reported in Spain11 and Italy.9,10
Kasera et al.4 showed sensitisation to legumes in 58.5% of patients with kidney beans, chickpeas and peanuts as the principal allergens in India. In Morocco, Ouahidi et al.8 reported that 23.4% of patients studied had positive values of IgE to peanuts. We have demonstrated that for dry white beans, 39% of adults had positive IgE values (data not shown).
The ELISA results after heat treatment of BBP showed a slight reduction in the recognition of these antigens by rabbit IgG for all temperatures studied. We recorded a maximum decrease at 70°C of about 22% after 60min incubation. However, under the same conditions, a very slight decrease was observed in the recognition by human IgE, with an average reduction of 12% for all patients. Gel electrophoresis showed the same protein banding profile before and after heating. This indicates that antigens recognised by IgG and IgE in the studied population were sequential sites. This finding is confirmed by data obtained in Italy where allergic reactions after ingestion of both raw and boiled broad beans by patients indicated that the recognised allergens were resistant to heating.9,10
For the enzymatic hydrolysis processing, many works have studied leguminous allergens leading to two possibilities, Firstly, a diminution of IgE binding to processed allergens has been observed for peanuts, soy products12,20 hazelnuts,21,22 and lentils.23 Other works have demonstrated a stability of allergens (soy, peanuts) after enzymatic hydrolysis.24,25
In our study, following enzymatic processing with pepsin, the binding of the IgG to hydrolysed BBP was highly diminished with a noticeable 55% decrease in the first 30min of treatment. Indeed, the electrophoretic profile showed an important attenuation of all protein bands after pepsin hydrolysis. This indicates that most of the sites recognised by rabbit IgG were cleaved by pepsin. Conversely, the recognition of BBP by human IgE increased greatly, meaning that new allergenic sites have been unmasked after BBP-proteolysis.
By combining the two treatments (heat and pepsin), we obtained an average reduction in the recognition of BBP by IgE of about 16% for all patients. This indicates that heating induced the denaturation of BBP which further increased the extent of enzymatic digestion.
From the results of this study, it was concluded that there was a strong sensitivity of the Moroccan population to broad bean proteins even after their digestion by pepsin, but this immunoreactivity can be slightly reduced when the enzymatic hydrolysis is preceded by heating.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentRight to privacy and informed consent. The authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestNo conflict of interest.
We thank the University Hospital centre of Fez as well as the medical laboratories of Fez and Casablanca for their help in recruiting patients. We thank also Professor Ettayebi Mohammed, for his help in revising manuscript.