Atopy patch tests (APT) have been introduced as a valuable tool for the diagnosis of food allergy. However, interpretation of the readout of APT requires further clarification.
ObjectiveTo investigate the accuracy of APT in identifying atopic sensitisation to hen's eggs (HE), cow's milk (CM), soybean and wheat in Chinese children with atopic dermatitis (AD) aged less than two years and to evaluate skin signs of APT for accurate diagnosis of food allergy.
MethodsAPT was performed and food allergy confirmed by open oral food challenges with HE, CM, soybean and wheat in 150 Chinese AD children aged less than two years. The sensitivity, specificity, positive (PPV) and negative (NPV) predictive values, positive (LR+) and negative likelihood ratio (LR−) of APT were calculated.
ResultsErythema and infiltration were not sufficiently indicative of a positive APT. The PPV increased with the appearance of indurations and the number of papules. The true positive APT rate increased from scores of + to +++. The PPV and specificity were 100% while APT scores of +++ were obtained with HE, CM and wheat. The sensitivity of APT with HE, CM, soybean and wheat allergy ranged from 59.6% to 90.5%, while the specificity ranged from 82.1% to 92.4%.
ConclusionThe APT is a suitable method for the diagnosis of AD in Chinese children aged less than two years with food allergies. Erythema and infiltration are not sufficient indicators of APT positivity. The PPV increases with indurations and the number of papules.
Atopic dermatitis (AD) is a T-cell-mediated chronic inflammatory skin disease, associated with hyperreactivity to environmental antigens, such as food allergens and aeroallergens.1 Moreover, food allergy occurs in the early years of life, up to three years of age, when tolerance to food is established and sensitisation to aeroallergens occurs.2 Up to 40% of children with AD also have clinically relevant food allergy,3 with the most commonly involved foods being milk, egg, fish, wheat, soy and peanut, which account for 90% of positive clinical responses.4 Therefore, accurate and timely identification and avoidance of the relevant allergens are critical for the effective treatment of AD in infancy and early childhood and prevention of relapse.
It is generally accepted that oral food challenges (OFC) are the gold standard for the diagnosis of food allergy. However, such tests are time-consuming, difficult to perform in the clinic, troublesome for the patient and can be associated with the risk of severe allergic symptoms. Therefore, laboratory-based diagnostic tools are required to minimise the frequency of double-blind placebo-controlled food challenges (DBPCFC). Skin prick tests (SPT) and the measurement of specific serum IgE (sIgE) are considered to be good tests for the diagnosis of immediate food hypersensitivity, although these techniques are not suitable for the identification of allergens in delayed-onset reactions.5 There are no standardised diagnostic levels for sIgE, which has different diagnostic criteria for various foodstuffs and are age-dependent.6 The SPT, which is simple to conduct, with no requirement for blood sampling, is an inexpensive and widely used method for the assessment of IgE-mediated food allergy. However, positive SPT results are based on allergen binding to specific IgE antibodies attached to mast cells, leading to the release of inflammatory factors. Therefore, positive SPT responses indicate only the presence of allergen-bound antibodies attached to mast cells. In other words, these tests reflect sensitisation, but not clinical allergy.
Recently, atopy patch tests (APTs), which are devoid of any side-effects were introduced as part of the diagnostic work-up for the diagnosis of non-IgE-mediated food allergy, seen in conditions like AD or digestive disorders.7–10 Atopy patch tests may aid in the early diagnosis of food allergy in preterm, while SPT and sIgE tests are negative.8,11 Furthermore, the detection rate is increased when APT are used in parallel with an SPT or sIgE tests.
For a long time, the use of APTs in clinical practice was limited by subjective interpretation and intra-observer variation and differences in the diagnostic accuracy of these tests between studies. Eventually, in 2006, the European Task Force on Atopic Dermatitis (ETFAD) proposed a standardised method for the interpretation of APTs in children with AD, based on graded qualitative measures (−, +, ++, +++ and ++++) recorded following the manifestation of erythema, vesicles and the number of papules.12 Subsequently, research into the diagnostic accuracy of APTs designated reactions graded as + and above as positive APT results. However, the clinical relevance of different grades of positive APT reactions is unclear. Moreover, data describing the effect of the severity of skin signs on APTs are scarce.
In this study, APTs were performed with hen's egg (HE), cow's milk (CM), soybean and wheat in Chinese AD children aged less than two years and food allergy was confirmed by OFC. The diagnostic properties of the different classifications of APT results were prospectively evaluated in relation to the outcome of controlled food challenges in order to validate the clinical relevance of different grades of APT reactions. Consequently, the influence of the severity of skin signs on the diagnostic accuracy of APTs was evaluated in children with AD. Furthermore, the value of APT skin signs in the diagnosis of food allergy without OFC was investigated.
Materials and methodsPatientsFrom November 2008 to July 2011, 150 children (56 females and 94 males) with suspected food allergy and AD and fulfilling the criteria of Hanifin and Rajka,13 were enrolled in this study. The ages of these patients ranged from 3 to 24 months (mean 9 months). Severity scoring of AD (SCORAD), reflecting the severity of eczema, ranged from 32 to 81 (mean SCORAD±SD=47.7±15.0). SCORAD includes topography items (affected skin area), intensity criteria (erythema, oedema, crusts, excoriations, lichenification and xerosis) and subjective evaluations (intensity of itch and loss of sleep).14 Seventy-seven children had moderate AD (SCORAD 25–50) and 73 children had severe AD (SCORAD>50). In addition, 20 healthy children without eczema, asthma, allergic rhinitis and AD (10 males and 10 females) aged 3–24 months (mean±SD=8±5 months) were enrolled as the control group.
All of the patients and the healthy children were referred to the Paediatric Dermatology Outpatient clinic of the Children's Hospital of Chongqing Medical University. During the study, each child was evaluated by the same investigator. Patients with systemic diseases, acute infectious diseases and autoimmune diseases were excluded from the study. Children did not take oral immunosuppressive drugs, including oral corticosteroids, for at least one month before the test. Oral antihistamine drugs and topical treatment of the back with corticosteroids were discontinued for at least seven days before the test. The study was reviewed and approved by the Institutional Review Board of The Children's Hospital of Chongqing Medical University and informed consent was obtained from the parents of the children.
Study designOn an in-patient basis, the children with the symptoms suggesting food allergy were challenged in an open manner. APT were performed in all AD patients and 20 healthy children before the oral food challenges with the identical native fresh allergens, including CM, HE, soybean and wheat.
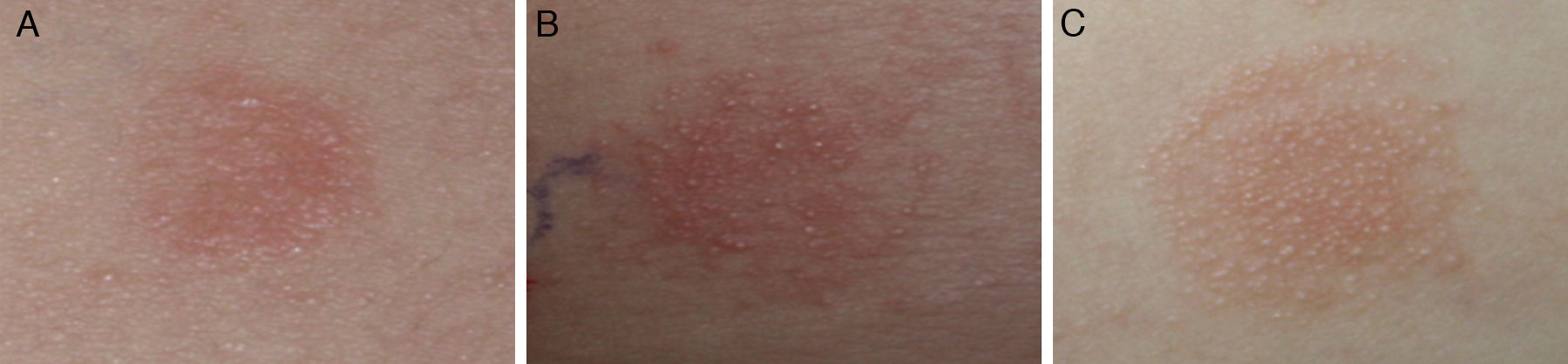
Atopy patch testAtopy patch tests were performed according to the ETFAD protocol.12 The patch test formulation was prepared fresh each day with one part of petrolatum and two parts allergen powder: cow's milk (milk powder containing 3.5% fat), fresh egg (white and yolk), soybean (raw soybean, crushed and mixed as a powder) or wheat powder. The formulations were then placed in 12mm Finn Chambers (Epitest Ltd., Oy, Tuusula, Finland) on uninvolved areas of the patient's upper back, which was prepared to ensure that the area was free of ointments and excessive sebum. Petrolatum was used as a negative control. The occlusion time was 48h and the result was read 24h after the removal of the Finn Chambers. The APT results were graded according to ETFAD standards: no reaction or erythema without infiltration (−), erythema and infiltration (+), erythema and few papules (++), erythema and many or spreading papules (+++), erythema, papules and vesicles (++++).
Elimination dietPrior to challenges, the patients were restricted to a milk, egg, soybean and wheat elimination diet for at least two weeks. During the elimination period, contact was maintained between the parents of the study subjects and a dietician. The mothers of the breastfed infants were instructed to abstain from foods containing milk, egg, soybean and wheat proteins during the elimination period. Formula-fed infants were placed on an extensively hydrolysed formula.15
Oral food challengesAfter a successful elimination diet, challenges with individual foodstuffs were performed in patients without a history of severe adverse reactions to food, such as shock, asthma and collapse. For the nursing mothers, foodstuffs were individually reintroduced into the diet after an interval of at least seven days. OFC were performed on children who were not breastfed in an open clinical setting, starting with those foodstuffs to which the child had the weakest or negative skin test results.
Milk and soybean milk were administered in successive doses of 5, 10, 50 and 100ml.7 Wheat powder dissolved in water (total amount 10g of wheat protein) was given in successive doses of 0.1, 0.3, 1.0, 3.0, 10.0, 30.0ml.5 and the oral challenge test was started with a quarter of a boiled egg followed by a gradual increase to a whole boiled egg.7 The time interval between each dose was 20min. Full emergency equipment and drugs (epinephrine, antihistamines, steroids) prepared according to patient weight were at hand. Challenges were discontinued if clinical symptoms were observed or the highest dose was reached. The child was observed for 2h prior to discharge and challenges were continued at home for the following six days. If any positive clinical symptoms occurred during this time or on the seventh day after challenge, the parents were told to contact the outpatient service and the children were examined by the author.
The food challenges were scored as positive if at least one of the following clinical reactions were noted: wheal, angio-oedema, SCORAD increase of at least 10 points, vomiting, diarrhoea, cough, asthma and shock.7 A reaction within 2h after challenge at the highest dose was regarded as an early reaction, while late reactions were regarded as those for which symptoms occurred later than 2h, but within six days.
Statistical analysisStatistical analyses were performed using SPSS for Windows 13.0. Two by two tables were used to calculate sensitivity, specificity, positive predictive values (PPV) and negative predictive values (NPV). Sensitivity was defined as the proportion of true positives detected, specificity as the proportion of true negatives detected, PPV as the proportion of symptomatic individuals among test positives and NPV as the proportion of non-symptomatic individuals among test negatives. Positive likelihood ratio (+LR) is the ratio of true positives to false positives, and negative likelihood ratio (−LR) is the ratio of false negatives to true negatives.
ResultsOutcome of OFCA total of 498 food challenges were performed with the four major foodstuffs in 150 children (138 with HE, 150 with CM, 123 with soybean and 87 with wheat). Positive open challenges were identified to HE in 92 (66.7%), CM in 94 (62.7%), soybean in 48 (39.0%) and wheat in 21 (24.1%). Multiple food allergy was detected in 33 AD patients. Of 255 positive challenge reactions, 17 (6.7%) were early reactions, including four vomiting and 13 urticaria, 137 (53.7%) were late reactions, all of which were manifested as eczema, and 101 (39.6%) were combined early and late reactions, 100 of which were combined eczema and perioral urticaria, and one case of combined vomiting and eczema. The main demographic and clinical characteristics of the study population are shown in Table 1. Asthma, airway obstruction and shock did not occur in any of the subjects during OFC.
Main demographic and clinical characteristics of the study population.
| Age | 3–24 months, mean 9 months | |
| Sex | 56 females, 94 males | |
| SCORAD | 32–81, mean 47.7±15.0 | |
| Mild (0–25) 0, moderate (25–50) 77, severe (>50) 73 | ||
| Family history of atopy | Eczema or AD | 2 |
| Asthma | 14 | |
| Allergic rhinitis | 35 | |
| Nickel contact dermatitis | 138 | |
| Deny | 12 | |
| Oral food challenges | HE 138, CM 150, soybean 123, wheat 87 | |
| Positive challenges | HE 92, CM 94, soybean 48, wheat 21 | |
| Multiple food allergy | HE+CM | 17 |
| HE+soybean | 7 | |
| HE+CM+soybean | 4 | |
| HE+CM+soybean+wheat | 1 | |
| CM+soybean | 3 | |
| CM+wheat | 1 | |
| Manifestation of clinical symptoms | Early reactions (<2h) | 17 |
| Vomit | 4 | |
| Urticaria | 13 | |
| Late reactions (2–24h 136, >24h 1) | 137 | |
| Eczema | 137 | |
| Combined early and late reactions | 101 | |
| Eczema+perioral urticaria | 100 | |
| Eczema+vomiting | 1 | |
Twenty patch tests were performed in the healthy control group and 150 in the test group. Positive APTs were not detected in the healthy control group. Erythema was observed in six of 150 AD patients following APT with petrolatum, but no infiltration and papule development. These patients were defined as APT negative and all suffered from severe AD. Sixty-eight of 150 (45.3%) APT results were assessed as positive for HE, 66 (44%) for CM, 47 (31.3%) for soybean and 24 (16%) for wheat. Of the 17 early reactions, APT positive results were detected in only six patients. Five manifested as infiltrated erythema, without papules and indurations. Only one patient exhibited infiltrated erythema and one papule at the HE allergen patch test site, but without indurations. This patient vomited 1h after the challenge with egg had a false positive APT result with soybean.
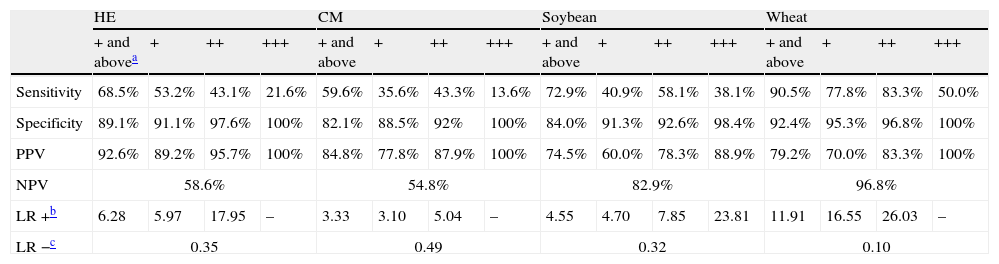
Value of the APT in the diagnostic work-up of food allergyAPT values of + and above were defined as clear-cut positive results. Of the children who developed positive oral food reactions, a positive APT result was found with HE in 63, CM in 56, soybean in 25 and wheat in 19. The lowest rate of false negative APT results was detected with wheat (2 of 21). Sensitivity, specificity, positive and NPV of APT are presented in Table 2.
Sensitivity, specificity, positive and negative predictive values, positive and negative likelihood ratio for APT in the diagnosis of HE, CM, soybean and wheat allergy in children with atopic eczema/dermatitis.
| HE | CM | Soybean | Wheat | |||||||||||||
| + and abovea | + | ++ | +++ | + and above | + | ++ | +++ | + and above | + | ++ | +++ | + and above | + | ++ | +++ | |
| Sensitivity | 68.5% | 53.2% | 43.1% | 21.6% | 59.6% | 35.6% | 43.3% | 13.6% | 72.9% | 40.9% | 58.1% | 38.1% | 90.5% | 77.8% | 83.3% | 50.0% |
| Specificity | 89.1% | 91.1% | 97.6% | 100% | 82.1% | 88.5% | 92% | 100% | 84.0% | 91.3% | 92.6% | 98.4% | 92.4% | 95.3% | 96.8% | 100% |
| PPV | 92.6% | 89.2% | 95.7% | 100% | 84.8% | 77.8% | 87.9% | 100% | 74.5% | 60.0% | 78.3% | 88.9% | 79.2% | 70.0% | 83.3% | 100% |
| NPV | 58.6% | 54.8% | 82.9% | 96.8% | ||||||||||||
| LR +b | 6.28 | 5.97 | 17.95 | – | 3.33 | 3.10 | 5.04 | – | 4.55 | 4.70 | 7.85 | 23.81 | 11.91 | 16.55 | 26.03 | – |
| LR −c | 0.35 | 0.49 | 0.32 | 0.10 | ||||||||||||
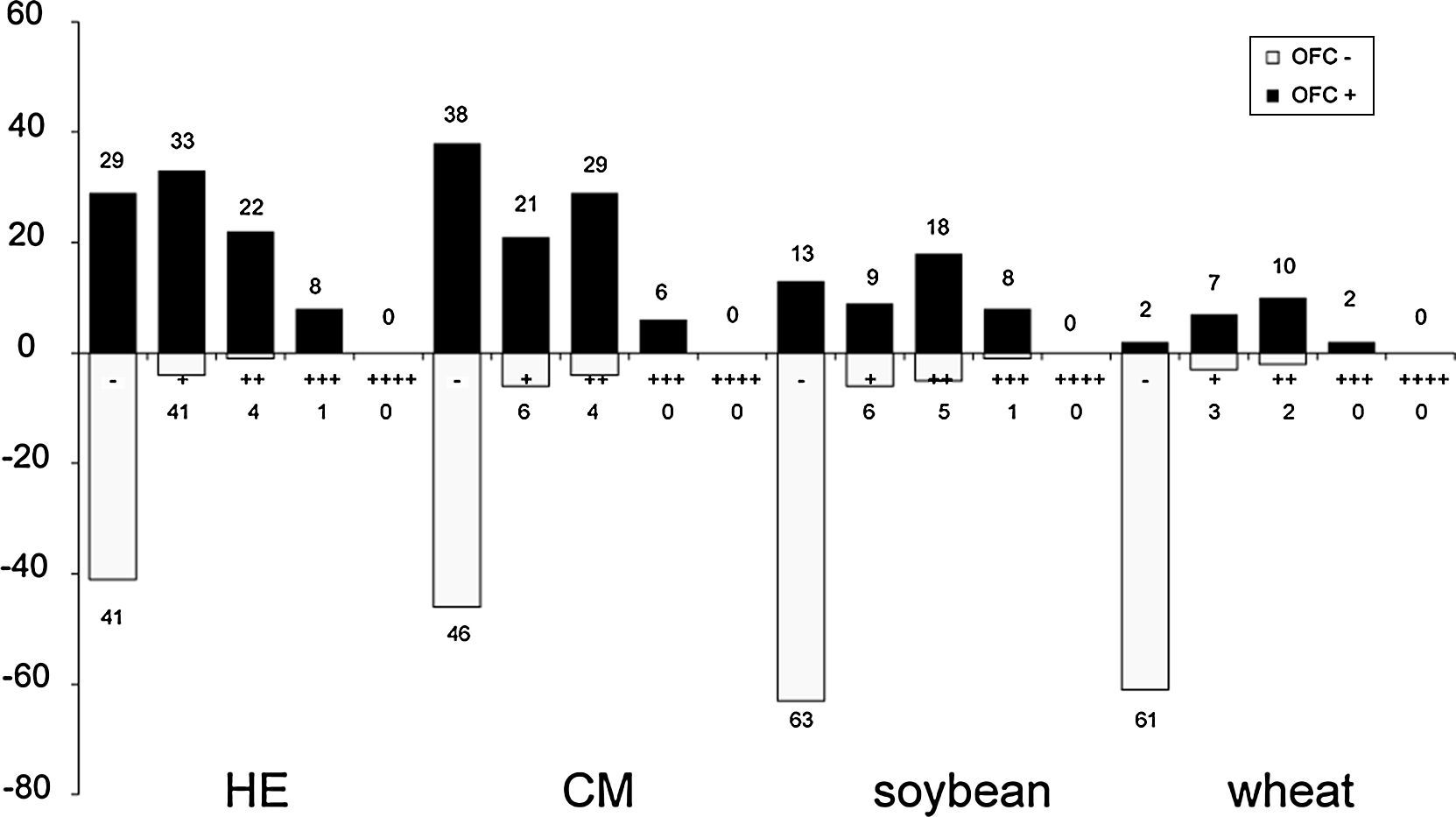
No cases of vesicles (++++) were found in the APT results obtained in this study. The qualitative readouts of positive APT results are shown in Fig. 1. Details of APT levels in the diagnosis of food allergies in AD patients are shown in Fig. 2. A tendency was observed towards a relationship between positive APT results and PPV. Data showed that specificity and PPV increased with increasing positivity of APT (Table 2). Both specificity and PPV were 100% when the APT with egg, CM and wheat was +++. Furthermore, the skin signs in all of these APT were erythema, infiltration, indurations and more than 10 papules. Moreover, one case of false positive APT with soybean, exhibited skin signs consisting of erythema, infiltration and no more than five papules, with no indurations.
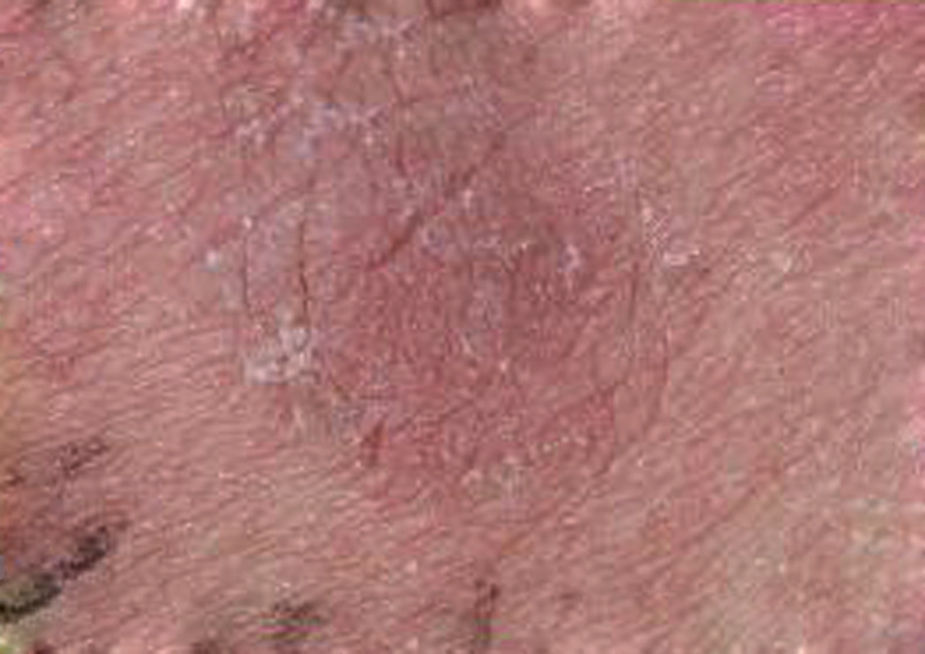
Furthermore, the appearance of an unusual reddish-brown macula with deep infiltration lasting for two weeks was observed at the positive patch site (Fig. 3). According to the ETFAD criteria, this may correspond with the + readout. The PPV was 100%. In addition, all the subjects were diagnosed with severe AD, with dry and scaly skin being the main clinical manifestation among these the patients. True positive APT results were confirmed in subsequent food challenges. All participants manifested with aggravated eczema and the reappearance of erythema and indurations was observed at the patch test site where inflammation had subsided.
Two false negative results were identified among 63 negative APTs with wheat. Of these patients, one was an eight-month-old boy, whose SCORAD was 53. However, erythema and infiltration were observed at the patch test site three days after the wheat challenge and remained for longer than two weeks.
DiscussionThis study was conducted in AD patients aged two years and younger diagnosed on the basis of open food challenges. To date, DBPCFC are recognised as the gold standard for the diagnosis of food allergy due to minimal involvement of subjective factors among researchers and subjects.16 However, the European Academy of Allergology and Clinical Immunology considers that the involvement of subjective factors is negligible among infants and children younger than three years of age and has approved the use of open food challenges in the diagnosis of food allergy.17 In our study, SCORAD calculation and the readouts of skin tests and OFC were performed by a single individual (the author) in order to minimise manual errors.
In this study, only 6 of 17 APT results were positive in diagnosing early reactions, and almost all the manifestations consisted of infiltrated erythema and without indurations, and only one case with a single papule. This result supports previous findings that APT does not confer any advantage in the diagnosis of early reactions.3,4 However, the value of this interpretation is limited by the small sample size of this study and much higher sample numbers are required to confirm this conclusion.
The absence of positive APT results in the control group supports the effectiveness of APT in diagnosis of food allergy in children with AD. Six of 150 patients presented with erythema without infiltration, indurations and papules at the site of patch tests with petrolatum, which suggested that a non-allergic response was stimulated and that petrolatum represented a suitable negative control. High specificity was observed in the calculated predictive capacity of APT for the four foodstuffs, ranging from 82.1% to 92.4%, while the values for sensitivity ranged from 59.6% to 90.5%. The values for the sensitivity and specificity of APT for the diagnosis of wheat allergy were both above 90%, which was consistent with previous studies.18,19 Therefore, APT was found to be an effective method for the diagnosis of wheat allergy in children with AD.
Our findings suggest that the presence of erythema and infiltration were not sufficient to define a positive APT, and the specificity (but not the sensitivity) was improved by the appearance of indurations and papules. Furthermore, the specificity and PPV increased with the number of papules.
In our study, no cases of vesicles (++++) were observed. It can be speculated that this can be accounted for by the study population. The rate of true positives increased with the degree of APT positivity, indicating that the rate of false positives decreases concomitantly and that the rate of correct diagnosis of food allergy is increased. Positive food challenges were found to correspond with APT results of +++ with HE, CM and wheat, thus indicating that food challenges are unnecessary for the diagnosis of food allergy in these cases.
Among the APT skin signs, the depth of infiltration and appearance of reddish-brown plaques were identified to confer diagnostic value. Typical manifestations of allergy include indistinct margin, crescendo, persistence, infiltration and papules, without bullae and necrosis. All these manifestations were detected in severe AD patients with egg and/or soybean allergy. This may be associated with serious damage to the skin barrier in these children, resulting in easier movement of egg and soybean antigens across this barrier due to higher lipid solubility compared with wheat and CM.
In patients with food allergy subjected to APT, it was observed that erythema and infiltration developed at the patch test site following oral challenges after APT pigmentation had subsided. It can be speculated that this procedure mimics the effects of foodstuffs that induce and aggravate eczema. Furthermore, this effect is similar to the development of fixed drug eruption, and therefore indicates that APT represents a suitable model for the investigation of fixed drug eruption.
The occurrence of side-effects during APT is rare. Contact urticaria and irritation reactions have been reported in small numbers of patients.5,20 A generalised relapse of AD and exacerbation of underlying asthma after APT has also been reported,18 although no cases of active sensitisation induced by APT have been described. In our study, a boy with severe AD tested negative in APT with wheat, although erythema and infiltration were observed at the patch test site after wheat challenge, and eczema was aggravated. This suggests that patch tests can result in sensitisation, while oral challenge can be manifested as clinical allergy.
In the review of the data from many studies of children with atopic dermatitis, many patients had a concomitant gastrointestinal reaction. And more than 80% of patients with delayed reactions of vomiting and diarrhoea after a food challenge had positive patch test to a food antigen. It suggests that patch testing might detect gastrointestinal food allergies. In recent years, many researchers found that atopy patch test is also a useful tool in the diagnostic work up of children with food-allergy-related gastrointestinal symptoms, such as Eosinophilic Oesophagitis and food protein-induced enterocolitis syndrome.9,21,22
Taken together, the findings of our study indicate that APT is a valuable tool for use in the diagnosis of food allergy in children with atopic eczema aged less than two years. It was observed that the rate of correct diagnosis of food allergy increased with the degree of APT positivity. However, the appearance of erythema and infiltration was not sufficient for the determination of APT positivity. The PPV increased with the appearance of indurations and the number of papules. Further investigation is required for the standardisation of APT to ensure correct application of this method and interpretation of the results.
Ethical disclosuresPatients’ data protectionThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestThe authors have no conflict of interest to declare.
We thank all the researchers and workers in Key Laboratory of Pediatrics in Chongqing for their technical assistance.