INTRODUCTION
There are flour derived from vegetable families not taxonomically related to cereals, that have been causatively incriminated in immediate hypersensitivity reactions. This is the case of sesame seed (Pedaliaceae) (1), buckwheat (Poligonaceae) (2, 3), and legumes (4-6). Grass pea (Lathyrus sativus), is a plant belonging to the leguminoseae family. Its flour is used in cattle feeding and also for human consumption in some countries. Lathyrism, a neurologic illness, has been associated with chronic ingestion of this legume.
We report a patient who suffered from rhinitis and asthma attacks shortly after grass pea flour (GPF) exposure in his work environment. We describe a new source of allergenic exposure to GPF, used in the industrial processing of hardwood flooring (parquet).
CASE REPORT AND DIAGNOSIS
We report a case of a 55 years old man, a nonsmoker, without any previous history of atopy, referred to our Allergy department with one year long history of rhinorrhea, paroxysmal sneezing, eyes and nasal pruritus, and wheezing dyspnea with coughing and chest tightness, symptoms which were related to his working activity. These symptoms developed immediately after exposition to GPF. The severity of these symptoms decreased markedly after he left the place, as in holidays and weekends, until he finally remained completely asymptomatic.
The patient had worked in direct contact with GPF for 15 years, making a mixture with water in order to spread it in the wooden panels floor to seal all the junctures. He had never eaten grass pea and he usually ate other legumes with good tolerance.
Grass pea flour extract for in vitro and in vivo tests
Commercially available GPF was defatted with several washes in acetone and extracted in phosphate buffered saline (PBS) through mixing 2 g of flour in 20 ml of PBS (pH 7.3) at laboratory temperature. After it had been stirred for 30 min the solution was centrifuged at 3,000 g for 10 minutes. The supernatant was centrifuged again and then the solution was passed through a Millipore filter (0.22 μ) for sterilization (Millipore, Molsheim, France), with a final concentration of 1/10 w/v. This solution was considered the undiluted extract. Ten fold dilutions were made for skin and bronchial challenge test. The protein concentration in the final non-dialysed extract as determined by Bradford assay (7) (Bio-Rad, Munchen, Germany) was 8.8 mg/ml.
A dialysed extract of GPF by an Spectrapor (standard cellulose dialysis tubing, molecular weight cut off 10 kDa) (Spectrum Medical Industries Inc, US) in PBS for 24 h at laboratory temperature was also obtained.
Skin prick tests
Skin prick tests (SPT) were performed with the allergy pricker lancet (Dome-Hollister-Stier) as described elsewhere (8). Histamine phosphate (10 mg/ml) served as positive control and NaCl (0.9 %) as negative control. The tests were read after 15 minutes. We considered a positive reaction if the wheal size was at least 3 mm diameter larger than negative control. SPT were done with our own GPF extract. Ten atopic and ten non-atopic controls were tested for SPT with GPF extract. Atopics were defined as having a positive clinical history and a SPT reaction more than 3 mm to at least one common allergen. SPT with a battery of commercially available common inhalants including grass pollen, an olive pollen, a mixture of tree pollen, outdoor-indoor molds, home dust mites and dog and cat dander (ALK-Abelló, Madrid, Spain) and cereal flours extracts including rice, rye, oats, soybean, corn, and wheat (Bial-Aristegui, Bilbao, Spain) were performed.
Specific IgE determinations
Specific serum IgE antibodies against GPF were measured by CAP enzimoimmunoassay for commercially available allergens according to the manufacturer's instructions (Pharmacia Diagnostics AB, Uppsala, Sweden).
Physiologic testing
Ambulatory peak expiratory flow rate (PEFR) in the workplace were recorded at 2 h intervals (except for sleeping periods) for two weeks at work and for two weeks away from work. These measurements were obtained while at work and at home, including weekends. This PEFR record was considered suggestive of occupational asthma if the mean PEFR was reduced on days at work or if there is a diurnal variability greater than 20 % that normalizes away from work (9).
Non-specific bronchial provocation test
The methacholine inhalation test was performed according to the method of Cockcroft (10), with some modifications. The aerosolized particles were generated by a continuous pressurized nebulizer, model De Villbis 646, with a fixed output of 0.28 ml/min.
Specific bronchial challenge test with grass pea flour
The patient was not exposed to GPF for one week previously to the challenge test, asymptomatic, and he did not take any medication which could affect the test results.
SPT with ten fold dilutions of GPF extract was previously performed to determine the safety of the procedure before the bronchial challenge test. The starting dose was the corresponding to a SPT with a 2-3 mm wheal diameter (1/100,000 v/v). A control challenge with PBS was carried out before antigen challenge When the patient demonstrated less than a 10 % fall in FEV1 with the PBS aerosol, he was considered suitable for challenge.
The patient was then admitted to Hospital and after obtaining his informed consent, he inhaled the aerosolized allergen for two minutes at tidal volume in progressively increasing concentrations at 30-minutes intervals. Pulmonary function measures of FEV1 and FVC were performed at baseline and at 5 minutes intervals for 30 minutes after each dose. Positive response was difined as > 20 % fall in FEV1 from baseline. Hourly FEV1 and FVC measurements were performed for the next 24 h (except for sleeping periods) following immediate positive response in order to evaluate late response. Two non-exposed asthmatic patients with similar nonspecific bronchial reactivity were also challenged after obtaining their informed consent.
SDS-PAGE and Immunoblotting
Gel electrophoresis of grass pea extract, was carried out in 4-15 % gradient polyacrylamide gels (Bio-Rad, Hercules, CA, USA) in both reducing and non-reducing conditions according to Laemli (11), using a SE250 Mighty Small mini-vertical Unit (Hoefer Scientific Instruments, San Francisco, CA, USA). Approximately, 40 μg of protein were applied to each lane. Rainbow molecular weight markers (Amersham, Buckinghamsire, UK) were used after denaturation. After the separation, one set of proteins was stained with Coomasie brilliant blue (Sigma, St Louis, MO, USA) and other set was transferred by electroblotting into PVDF sheets (Immobilon, Amersham) following the method of Towbin (12). Unreacted membrane sites were blocked for 1 h with TBS containing 3 % bovine serum albumin and 0.1 % Tween 20 (TBS-T-BSA). The membranes were incubated for 1 h at room temperature with patient or control's serum diluted 1/20. Washes were performed in TBS 0.1 % Tween. Afterwards, alkaline phosphatase-conjugated goat anti-human IgE (Tago, Burlingame, CA, USA), 1/200 in TBS-T-BSA, was incubated for 1 h at room temperature. Finally, the reaction was developed with nitro blue tetrazolium-5-bromo-4cloro-3indolyl phosphate (NBT-BCIP) as substrate.
RESULTS
Skin prick test with our own grass pea extract was positive with an immediate response, both with dialysed and non-dialysed extract. Ten atopics and ten non-atopics control subjects did not react to extract.
The patient did not have any positive reaction to either common inhalants or commercially available cereals flour extracts.
Specific IgE antibodies against grass pea flour (9.57 KU/l) was found in the patient's serum as determined by commercial CAP assays.
Sequential Peak expiratory flow rate in the workplace showed a diurnal variability greater than 20 % that normalizes during weekends. No significant variability was obtained on two weeks away from work.
Non-specific bronchial challenge test with metacholine revealed bronchial hyperreactivity with PC20 values of 6 mg/ml.
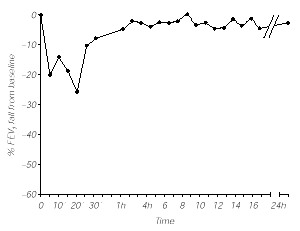
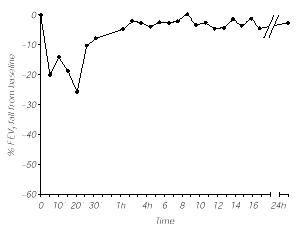
The specific bronchial challenge test with grass pea flour extract (1/100 v/v) elicited an immediate response, both clinically (rhinitis, cough, dyspnea, chest tightness) and spirometrically with a 20 % decrease in FEV1 at 5 minutes that was maximal at 20 min with a 26 % decrease from basal values and required salbutamol administration, returning to baseline levels 1 h later. No late reaction was observed (fig. 1). Control subjects showed no significant response after specific bronchial challenge test.
Figure 1.--Bronchial challenge test with Grass pea flour extract (1/100 w/v).
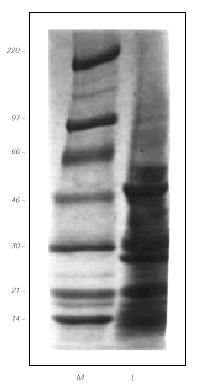
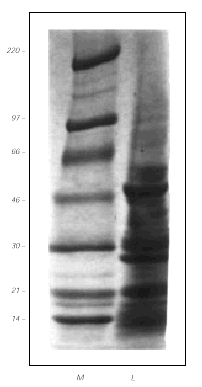
Coomasie staining after SDS-PAGE separation of the GPF extract, showed several protein bands in the range of 97 to 10 kDa (fig. 2). The main antigens had an approximated MW of 46, 28 and 21 kDa.
Figure 2.--Protein separation by 4-15 % gradient SDS-PAGE (in the absense of β -mercaptoethanol) and Coomasie blue staining of the gel. Left lane, molecular weight standards (in kDa). Right lane, grass pea flour extract (40 μg).
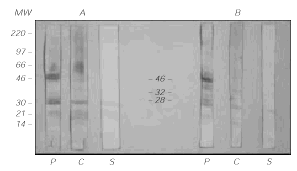
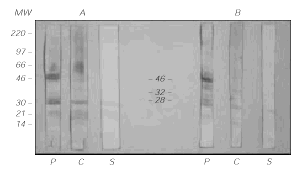
IgE immunoblotting (fig. 3) showed that three of the antigenic bands, of MW 46, 32 and 28 kDa, were specifically recognized by the patient's IgE and not by control sera or the secondary antibody.
Figure 3.--IgE-Immunoblotting of Grass pea extract (40 μg/lane) after protein transfer to PVDF membranes. In the left (A) panel, the samples were previously reduced with β-mercaptoethanol. In the right (B) panel, they were not reduced. P = patient, C = Control serum, S = Secondary antibody alone (goat anti-human IgE).
DISCUSSION
The clinical picture in our patient, with nasal and respiratory symptoms shortly after exposure to GPF at work and its improvement while the patient was away from work, strongly suggests the occupational nature of the patient's rhinitis and asthma. This conclusion is supported by the presence of a PEFR pattern with increasing variability in the workplace. The positive specific bronchial challenge test confirmed GPF was the ethiological agent in this case. The negative response to the highest concentration (undiluted) of GPF extract in two non-exposed asthmatic controls with similar nonspecific bronchial hyperreactivity rules out the existence of an irritant of pharmacologic mechanism. Moreover, the positive immediate response in SPT and the demonstration of specific serum anti-GPF IgE, strongly suggest the underlying type I hypersensitivity mechanism. The negative results in skin prick test and Western blotting to GPF in a group of unexposed normal and atopic subject confirm the specificity of these findings. The positive SPT with both dyalised and non-dyalised extract suggested the allergens had a MW higher than 10 kDa. The SDS-PAGE and immunoblotting confirmed this by demonstrating the existence of three main allergens of MW 46, 32 and 28 kDa. The MW of these allergens does not correspond to any of the GPF antigens identified by other groups: mitogenic lectin (49, 19 and 4 kDa) (19), putrescine synthase (55 kDa) (20) or arginine decarboxilase (220 and 38 kDa) (21).
Disulphide bonds and the tertiary structure of the allergens seem not to be critical in this particular case since neither reduction nor denaturation abrogate IgE recognition. Thus, the epitopes are probably encoded in the aminoacid primary sequence. Our patient will avoid eating grass pea since we can not assure that the enteric enzymes will destroy the allergenic epitopes.
Legumes have been frequently involved in type I hypersensitivity reactions, specially in food allergy resulting from their ingestion (13), but also from inhalation of particles in an aerosol way (14-16). A broad cross-reactivity has been demonstrated among different legumes (15-17), but seems not to have clinical relevance (18). Accordingly, our patient tolerates the ingestion of other legumes without any problems, and inhibition tests between grass pea and other legumes were considered unnecessary.
Gras pea (Lathyrus sativus) is mainly used for cattle feeding and its consumption by humans is discouraged and very limited due to the risk of lathyrism, a neurological disorder. However, it is still used in some countries, specially in times of famine. As far as we know, there is only previous report in the literature of bronchial asthma (not related to his working activity) to GPF by a type I hypersensitivity mechanism, in a 10-years-old child whose symptoms were related to GPF exposure in a bakery (6). This is the first time that occupational asthma by GPF is reported and also the first documented characterization of allergens in this legume.
Our study shows an alternative source of GPF sensitization, caused when it is used in industrial processing of parquet. GPF has to be taken into account as a possible causative agent of occupational asthma in these workers. Due to its multiple uses and different sources of exposition, GPF might constitute an important occupational allergen at least in some countries.