Cow's milk allergy (CMA) epidemiology seems to be changing over time, with an increase in prevalence and persistence. Our aim was to characterise a population of children with CMA beyond two years of age, followed up in a Paediatric Allergy Clinic at the tertiary care level.
MethodsRetrospective study of children with persistent CMA diagnosed from January 1997 to June 2006. Medical records were analysed regarding: clinical presentation, follow-up, treatment and acquisition of tolerance. Data analysis was performed using Excel 2007 for Windows.
ResultsSeventy-nine children were included, with mean age at first symptoms of 3 months. The symptoms were immediate in 93%, with cutaneous (87.3%), gastrointestinal (55.7%) and respiratory (25.3%) manifestations. During the follow-up period, 30% developed atopic eczema, 52% asthma and 35% rhinoconjunctivitis. A family history of atopy was identified in 53%. The majority presented increased serum total IgE (376±723 KU/l) and positive skin prick test (SPT) to cow's milk (CM) (79%). SPT to goat's milk was positive in 2/3 of cases. Fifty-five percent had at least one accidental exposure to CM (severe reactions in 6%). During CM elimination diet, 35% were initially given an extensively hydrolysed formula, 17% a soy formula, and 48% both. By the age of 10 years, 44% of children persisted with CMA.
DiscussionOur population of CM allergic children presented immediate symptoms with cutaneous expression in the majority. Severe reactions were common on accidental exposure. By the age of 10years, 44% maintained CMA, highlighting the importance of a multidisciplinary follow-up.
Cow's milk allergy (CMA), as any other type of food allergy, is classified as IgE and non-IgE-mediated, and is a form of food hypersensitivity. The latter also includes non-immunological reactions to food, such as adverse reactions to food contaminants and digestion disorders resulting from enzymatic defects (e.g. lactose intolerance, which is a differential diagnosis to consider when CMA is suspected).1,2
CMA affects about 2–3% of children in the first year of life. Cow's milk proteins are usually the first foreign food antigens introduced into an infant's diet, which partially explains the high prevalence of allergy to this food.3,4
Allergic reactions to CM may be immediate, i.e. developing within one hour after exposure to CM, which are likely to be IgE-mediated, or may be late reactions, i.e. developing a few hours after exposure, where usually cellular mechanisms are implicated.5 Among a wide range of possible CMA manifestations, the most common are cutaneous, gastrointestinal and respiratory symptoms. CMA diagnosis is based on a careful clinical history, skin prick testing to cow's milk (CM) and/or determination of serum specific IgE to CM, elimination of CM from the infant's diet (or the mother's diet in the case of breastfeeding) and oral CM challenge, as appropriate. Treatment consists of CM elimination diet and relief of acute symptoms when an accidental exposure occurs, according to a well-established and written treatment plan.
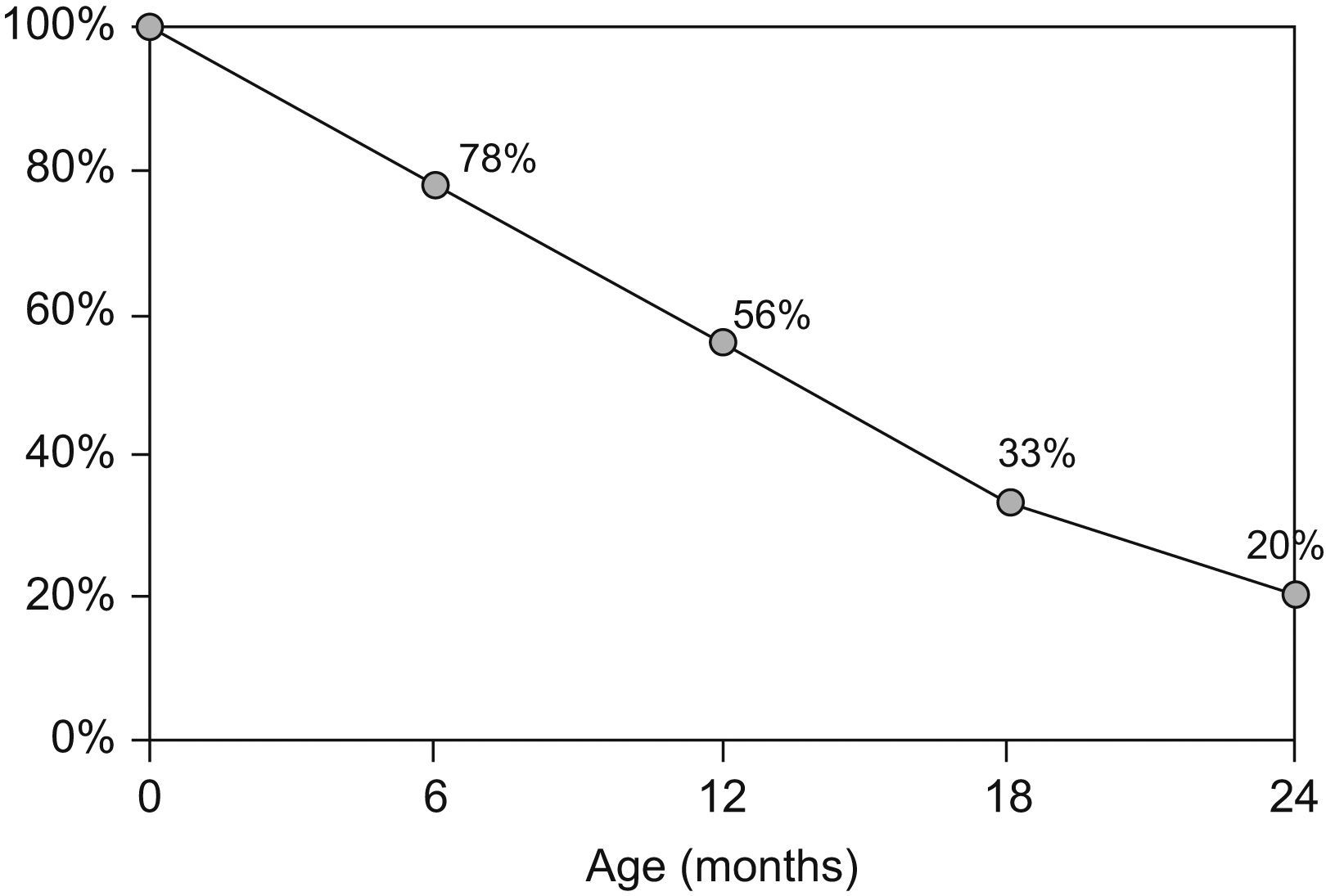
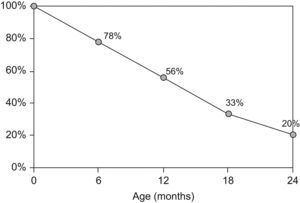
Although oral tolerance mechanisms are not completely understood, CMA is usually transient and tolerance has been reported in up to 80% of the children by the age of two (Figure 1).6 Children with non-IgE-mediated disease have been shown to develop tolerance earlier and more frequently than those with IgE-mediated CMA.7–9 However, allergy persists beyond the age of two in some children, in whom CM avoidance and specialised follow-up have to be continued.4,10 In spite of limited information on the acquisition of tolerance to CM in children with more persistent disease, recent data has shown a change in natural history of CMA, with increasing persistence until later age.7,11
Acquisition of tolerance to cow's milk during the first two years of age.6
This study focused on a population of Portuguese children with persistent CMA beyond the age of two, aiming at defining their clinical characteristics and the rate of CMA resolution over time.
MethodsChildren with persistent CMA beyond the age of two years followed up at our specialised Paediatric Allergy Clinic and diagnosed with CMA between January 1997 and June 2006 were included.
CMA diagnosis was based on the resolution of symptoms after avoidance of CM during four to six weeks and their recurrence on oral CM challenge. Patient's follow-up was usually started at the Paediatric Gastroenterology Clinic and continued at the Paediatric Allergic Clinic from the age of 2. At the age of 15, patients were discharged and followed-up in an Adult Allergy Clinic thereafter. They were submitted to Skin Prick Test (SPT) and/or determination of serum specific IgE to CM at diagnosis and over time to determine when to perform oral CM challenge. Tolerance was defined by a negative CM challenge followed by a regular consumption of an age-appropriate quantity of CM at home, without any symptoms.
IgE-mediated CMA was considered when clinical manifestations occurred within one hour after exposure to CM, and there was evidence of sensitisation to CM either by positive SPT to CM (defined by a wheal diameter greater than or equal to 3mm) and/or specific IgE to CM above 0.35KU/l. Whenever symptoms occurred beyond a period of one hour after exposure it was classified as a delayed manifestation. Related conditions were excluded.
Medical records were analysed for gender, age, clinical symptoms, accidental exposures during elimination diet, co-morbidities, personal history (prematurity, exposure to CM formula during the neonatal period, age of weaning), family history of atopy and CMA, results of allergy and related tests (peripheral eosinophil count, serum total IgE, specific IgE to CM and SPT to CM), treatment and acquisition of tolerance.
Data was analysed using Excel 2007 for windows.
ResultsFrom January 1997 to June 2006 (9.5 years) seventy-nine (n=79) children with persistent CMA beyond the age of 2 years, 52% female, were followed up at our Paediatric Allergy Clinic.
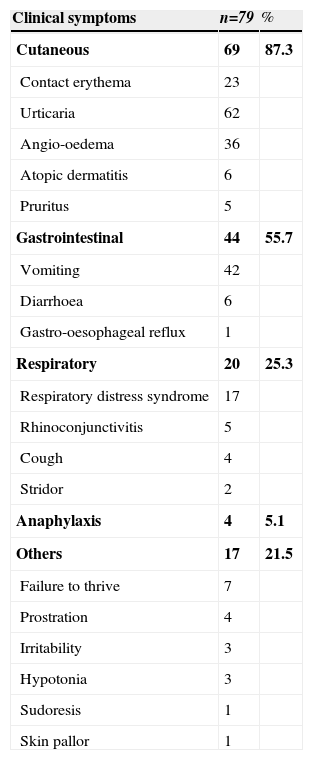
The mean age of onset of CMA was three months (standard deviation of 2 months). Immediate allergic symptoms were observed in 93% of children and the most common were urticaria (78.5%), vomiting (53%), angio-oedema (45.6%) and contact erythema (29%). Seven per cent had delayed manifestations (one hour after exposure) mainly prostration (5%), diarrhoea (7.6%) and failure to thrive (8.9%). Overall, the cutaneous manifestations were the most common (87.3%), followed by gastrointestinal (55.7%) and respiratory (25.3%) symptoms. Anaphylaxis occurred in 5.1% of cases (Table 1). More than one system was involved in 63% of children and the most common combination (48%) was concomitant gastrointestinal and cutaneous symptoms.
Frequency of clinical symptoms at CMA presentation
| Clinical symptoms | n=79 | % |
| Cutaneous | 69 | 87.3 |
| Contact erythema | 23 | |
| Urticaria | 62 | |
| Angio-oedema | 36 | |
| Atopic dermatitis | 6 | |
| Pruritus | 5 | |
| Gastrointestinal | 44 | 55.7 |
| Vomiting | 42 | |
| Diarrhoea | 6 | |
| Gastro-oesophageal reflux | 1 | |
| Respiratory | 20 | 25.3 |
| Respiratory distress syndrome | 17 | |
| Rhinoconjunctivitis | 5 | |
| Cough | 4 | |
| Stridor | 2 | |
| Anaphylaxis | 4 | 5.1 |
| Others | 17 | 21.5 |
| Failure to thrive | 7 | |
| Prostration | 4 | |
| Irritability | 3 | |
| Hypotonia | 3 | |
| Sudoresis | 1 | |
| Skin pallor | 1 |
During the follow-up period, 30% of children developed atopic dermatitis, 52% asthma and 35% allergic rhinoconjunctivitis. Sensitisation to airborne allergens was observed in 74% and to other food allergens in 61% of children. Nevertheless, only 32% of children with sensitisation to foods other than CM had true allergy to the same food, the most common being egg and fish allergies.
Eighty-one percent of the studied children were given cow's milk formula in the neonatal period. Age of weaning was on average three months (standard deviation of 25 days). A family history of atopy was present in 53% and of CMA in 7% of children.
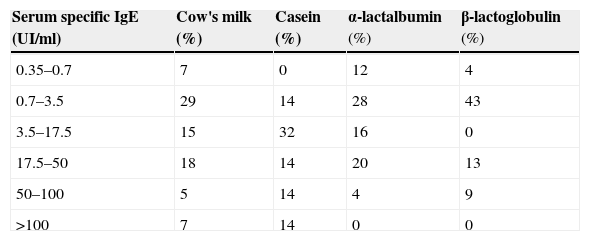
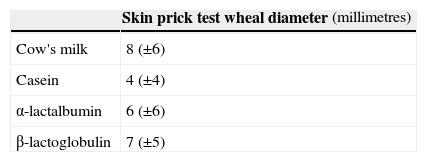
The majority had eosinophilia (56%) and raised serum total IgE (376±723 KU/l). Specific IgE to CM was positive at diagnosis in the majority of children (Table 2). SPT to CM was positive in 79% of children, to casein in 80%, to α-lactalbumin in 72% and β-lactalbumin in 83%, with wheal diameter of 8±6mm to CM, 4±4mm to casein, 6±6mm to α-lactalbumin and 7±5mm to β-lactalbumin, respectively (histamine positive control 6±2mm) see Table 3. SPT to goat's milk was positive in two thirds of the cases (wheal diameter of 5±6mm).
During the follow-up period, the children were subjected to four oral food challenges (OFC) with CM in average and the manifestations were immediate in the majority of cases (83%).
Complete CMP avoidance was recommended to all children with persistent positive oral food challenge based on parents and caregiver's education (the importance of CM elimination diet was carefully explained and detailed written information provided, the parents or carers were taught to read all the labels). During cow's milk elimination diet, 35% of children were given, initially, an extensively hydrolysed formula, 17% a soy formula and 48% both, at different times. SPT to soy was positive in 14% of children; however, clinical allergy to soy was confirmed on OFC in only one child. Three cases of allergy to extensively hydrolysed formulas (5%) were reported and confirmed on OFC. In these children amino acid-based formula was given as an alternative. Accidental exposures to CM occurred in 55% (40/73) at least once, with severe symptoms such as anaphylaxis in 10% (4/40) of cases. Auto-injectable adrenaline was the recommended medication in these situations.
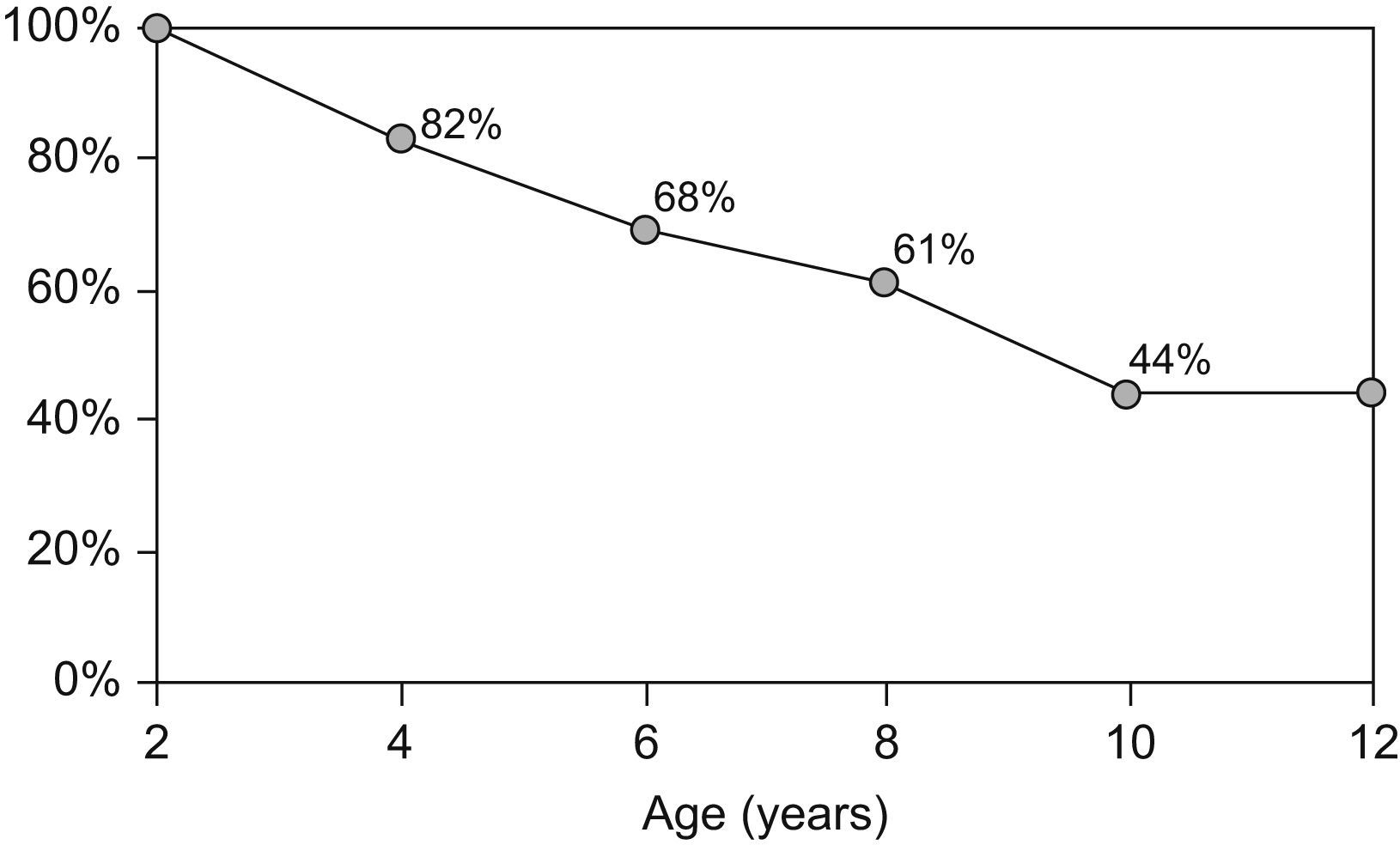
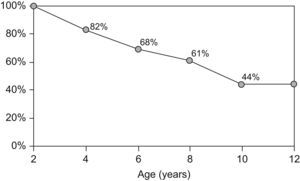
By the age of four, 17.7% of children had outgrown their allergy, 31.6% by the age of 6, and 39.2% by the age of 8. Forty-four percent of children persisted with CMA at the age of 10 years (Figure 2).
DiscussionAccording to recent studies performed on different populations, CMA epidemiology seems to be changing, with a greater prevalence of CMA at older ages.11–14 However, there is limited information available in this field. In this study, we characterised a population of Portuguese children with persistent CMA beyond the usual age of tolerance acquisition and compared it with other populations, as described in literature.
CMA was equally prevalent in both genders in our study population, where 52% of CMA children were female, as usually described. Nevertheless, some authors describe milder male gender dominance, which may be a result of greater prevalence of allergic disease in males until adolescence.12
CMA symptoms usually start within a week after the introduction of CM in the infant's diet; therefore they may vary in different populations and from child to child. In our study the first symptoms started by the age of three months, which was coincident with the mean age of weaning in all children.
Our study population showed a greater prevalence (93%) of immediate allergic manifestations, possibly IgE-mediated CMA. The most common manifestations were cutaneous, such as urticaria and angio-oedema, followed by gastrointestinal and respiratory symptoms. They commonly affected more than one organ, as observed in similar studies where IgE-mediated CMA was equally prevalent.7,12,15
In our study, SPT to CM and to CM proteins showed similar results, highlighting the limitations of their isolated use in the follow-up of CMA children.
Despite being the gold standard for the diagnosis of food allergy, OFC is a time-consuming, laborious technique, and not without risk. In an attempt to overcome this and to help determine when to perform OFC to assess the acquisition of tolerance, some authors have suggested quantification of serum specific IgE and skin prick test to CM to predict the acquisition of tolerance.6,10,14,16 These authors point out that the ones who achieve tolerance earlier have lower levels of CM specific IgE and smaller wheal diameters on SPT to CM.14
The high number of OFC performed in our study was justified by persistence of allergy until later ages. In addition, performing SPT and specific IgE quantifications were not part of the initial diagnosis and follow-up in many cases, which may be a limitation in our study. In this group of children, symptoms developed during the oral challenge were similar to the ones presented at the time of diagnosis.
Once CMA is diagnosed, it is important to identify the presence of risk factors for severe allergic reactions, such as previous life-threatening allergic reactions and/or the presence of uncontrolled asthma.17 The high number of accidental exposures during CM elimination diet in our patients, with severe adverse reactions in some cases, points out the need for a better education of patients and their families in order to maintain strict CM avoidance. A careful selection of “allowed” and “not allowed” foods, a written treatment plan and the education of school staff for correct administration of emergency medicine, such as auto-injectable adrenaline, are extremely important.12,18
The persistence of CMA implies a multidisciplinary and specialised follow-up. Special attention should be given to adolescents, who are more at risk for severe reactions, and to adequate intake of Vitamin D and calcium in all age groups, but especially in infants, to prevent future co-morbidities.17,19
In our study, 81% of newborns were given a CM formula in the Maternity Hospital. Neonatal exposure to CM proteins contributes for the sensitisation to CM. Seven percent of our children had CMA family history and 57% had referred atopic personal and family background. Several other studies revealed a high prevalence of atopic family history in groups of children with persistent CMA.13,20,21
The majority of children, in our study, developed other allergic diseases during the follow-up period. Atopic dermatitis is more prevalent in early ages and its prevalence decreases over time, which contrasts with symptoms of wheezing and rhinoconjuntivitis, whose prevalence increases with age as we observed in our population (data not shown). This is the so-called “allergic march”. Later tolerance has been related to the development of asthma, rhinoconjuntivitis, atopic dermatitis and other food allergies.7,8 A Portuguese study concluded in 2005 revealed a greater incidence of asthma in groups with persistent allergy to CM, highlighting the importance of a close follow-up of these children.22
The European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the American Academy of Paediatrics (AAP) recommend extensively hydrolysed formula for the first-line treatment of children with CMA. Soy milk is a cheaper alternative, but it is not recommended before six months of age, especially for infants with non-IgE mediated CMA.21,23,24 In our study, during CM elimination diet, both formulas were given as an alternative to CM, but fewer adverse reactions were observed in children on soy milk as compared to extensively hydrolysed formula (5% vs 2%). In case of adverse reactions to extensively hydrolysed formula (fortunately rare, but occurring in 5% of our children), amino acid-based formula was given as an alternative.21,23,25
Goat's and sheep's milk cross react with CM (SPT to casein were positive in 2/3 of children in our group) and are not appropriate alternatives to CM in CMA children.21,23
After the age of two, we observed a progressive acquisition of tolerance. Nevertheless, by the age of 10, 44% of the children with CMA beyond 2 years of age had persistent allergy and needed to maintain the avoidance of dairy products. This represents an overall persistence of 9%.
In summary, this group of children with persistent CMA was characterised by a family history of atopy and immediate symptoms after CM exposure, mostly cutaneous. The majority developed other atopic conditions over time, namely asthma, rhinoconjuntivitis, and other food allergies.
In the light of a change in the natural history of CMA, the persistence of CMA to later age implies a close and multidisciplinary follow-up over time. Special attention should be given to education of patients and their families in order to prevent accidental exposures.
Conflict of interestThe authors have no conflict of interest to declare.