Assessment of asthma with a control test has been suggested as a relevant approach in recent years. However, no biomarker of systemic inflammation has been included in the assessment of asthma control.
ObjectiveTo evaluate plasma paraoxonase (PON1), total oxidant status (TOS), and total antioxidant status (TAS) levels in children with asthma according to the disease control, and the performance in the identification of uncontrolled patients.
MethodsStable asthmatic children (n=85) and healthy controls (n=55) were recruited for this study. Blood samples were collected for plasma PON1, TOS, and TAS measurements. Any contributing factors that may affect plasma PON1, TAS, and TOS levels were excluded from both groups. The diagnostic potential of these measures was evaluated using receiver operating characteristic (ROC) analysis.
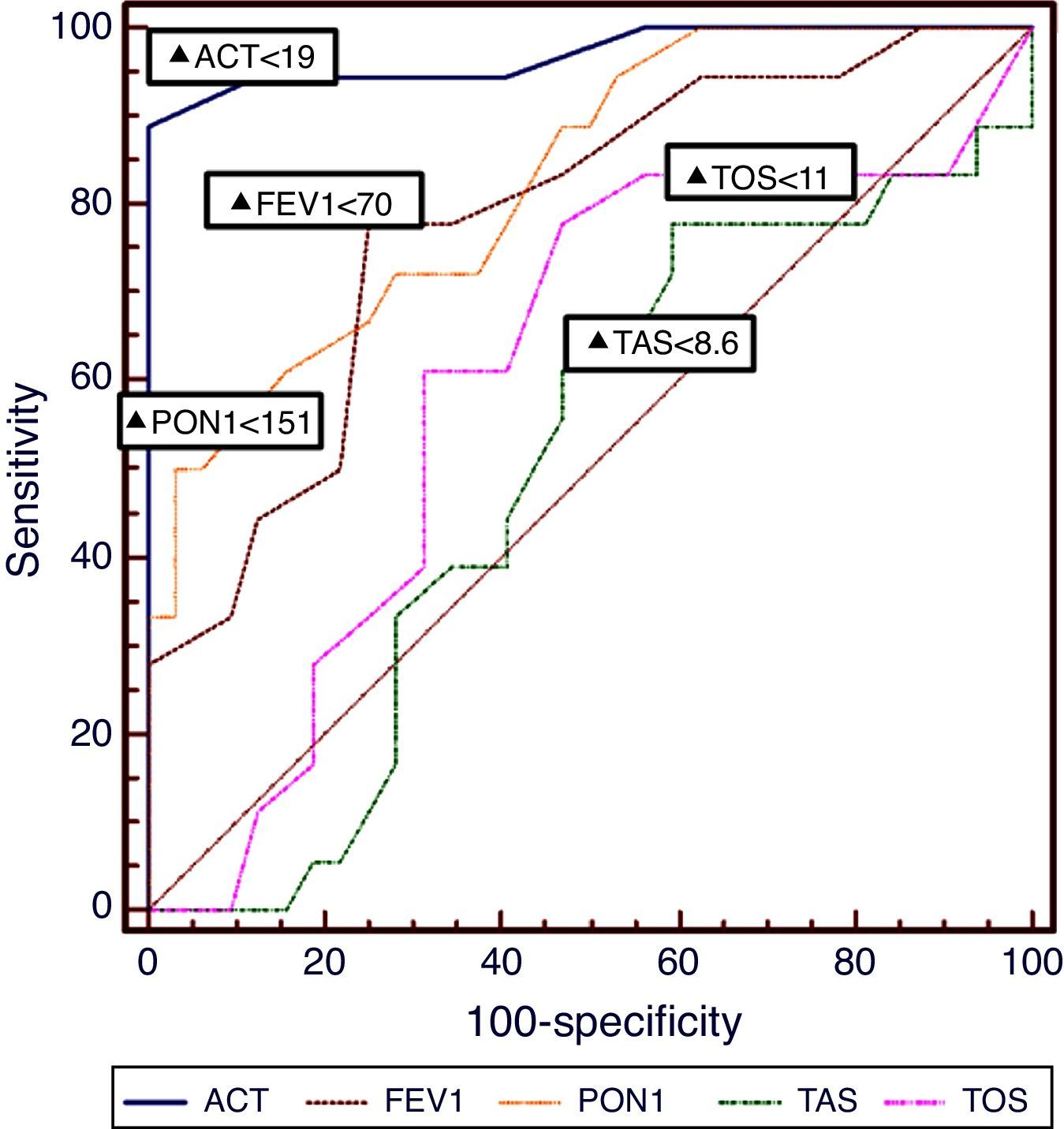
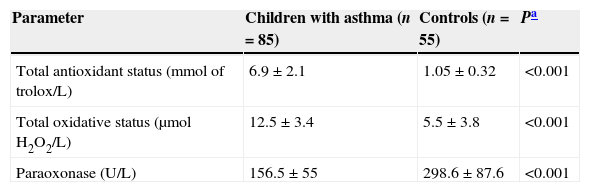
ResultsComparing the asthmatic children with the control group, plasma TAS and TOS levels were significantly higher (TAS; 6.9±2.1, 1.05±0.32, P<0.001, and TOS; 12.5±3.4, 5.5±3.8, P<0.001, respectively) and PON1 level was significantly lower (156.5±55, 298.6±87.6, respectively, P<0.001) in the asthmatic group than controls. In ROC analysis, PON1 presented an AUC 0.679 and TOS presented an AUC 0.645 for the identification of uncontrolled asthma, respectively. Asthma Control Test (ACT) presented an AUC of 0.972 for the identification of uncontrolled asthma.
ConclusionPON1 and TOS levels may be systemic markers of uncontrolled asthma in children. Combined use of these two biomarkers with asthma control test may identify patients with uncontrolled asthma in children.
Although the large surface area and high exposure to atmospheric oxygen increase the lungs’ susceptibility to oxidative injury, these organs are equipped with strong defences to counteract the oxidative insult (e.g. asthma is a chronic inflammatory lung disease that is worsened by increased oxidative stress).1,2 Increased levels of reactive oxygen species (ROS) such as hydroxyl radicals, superoxides and peroxides can lead to increased airway reactivity and secretions, and increased vascular permeability, which collectively augment the existing inflammation that is a hallmark of asthma.3,4 The mechanisms responsible for both remodelling and the inflammation are incompletely understood, but some clinical studies indicate that inflammation is related to disease severity.5,6 Despite longstanding use, the classification of severity is no longer recommended except in newly diagnosed patients; instead, the assessment of asthma control tests is reported as a relevant approach according to recent guidelines.7 Ideally, asthma control tests should include not only patient-reported clinical manifestations of the inflammatory process, but also laboratory markers that indicate inflammation and pathophysiological features of the disease as well. Subclinical inflammation may precede actual clinical impairment so that conventional measures may be insufficient to detect the inflammatory component of adverse health effects. Therefore, there is a need for objective evaluation of asthma symptoms and control, suggesting a possible role for biomarkers of airway inflammation. Unfortunately, objective measurement cannot be performed due to its prohibitive cost and the unavailability of confirmed markers or measures that definitively indicate inflammation.
Oxidised low-density lipoprotein (Ox-LDL) is a parameter closely associated with atherosclerosis. It is also suggested to be involved in the aetiology of many other diseases (e.g. chronic inflammatory diseases of the airways and includes other Chronic Obstructive Pulmonary Disease (COPD) diseases).8,9 Leukotrienes, known to be involved in asthma pathogenesis, are synthesised from arachidonic acid (AA) through the action of lipoxygenase.10 Because the leukotriene pathway is stimulated by ROS upon exposure to AA, a relationship may exist between LDL formation and the development of Asthma.11 Paraoxanase (PON1) is another component, which is known to retard the oxidation of LDL by preventing the generation of lipid peroxides.12 Serum PON1 is synthesised mainly by the liver that circulates serum in association with high-density lipoprotein (HDL).13 Additionally, it contributes to detoxification of organophosphorous compounds, including the pesticide paraoxon.14 In this study, aside from the members of lipid and lipoprotein profile, PON1 enzyme activities were determined in children with asthma because of their close association with lipoprotein metabolism as well as their antioxidant property.15 The aim of this study was to evaluate plasma PON1, total oxidant status (TOS), and total antioxidant status (TAS) levels in atopic asthmatic children and to investigate the correlation with the atopy, level of disease control and the frequency of attacks.
MethodsSubjects and study designWe conducted a prospective case control study among 85 children diagnosed with stable mild-to-moderate atopic asthma from the authors’ Paediatric Allergy-Pulmonology outpatient clinic and who were enrolled consecutively between October 2009 and December 2010. The diagnosis and severity of asthma were defined according to GINA16 guidelines. Children were defined as asthmatic according to the following criteria: (a) recurrent episodes of at least one symptom of asthma, including cough, wheezing, breathlessness, and chest tightness; (b) an improvement of at least 12% in baseline forced expiratory volume in one second (FEV1) after bronchodilator use; (c) a total serum Ig E level of over 52IU/mL determined by direct chemiluminescence, and a positive skin test for at least one allergen. Informed consent was given by the family of the patients.
Eighty-five subjects, aged 7–14 years, with stable asthma who had been attending the Paediatric Allergy Unit of Medical Faculty of Bezmialem Vakif University were recruited for this case-control study. Exacerbation of asthma was defined as episodes of progressive increase in shortness of breath, cough, chest tightness, or combination of these symptoms. After selecting the group, age, gender, duration of asthma, lung function tests, the frequency of attacks per year, day-time symptoms, the need for rescue treatment, and the medication used were all recorded. A family history of atopy was considered positive if atopy was present in parents and/or siblings (bronchial asthma, allergic rhinitis, atopic dermatitis). The patients also underwent skin-prick tests. If a wheal response of ≥3mm in diameter developed after 15min to one or more of the allergens in the presence of both negative (0.9% saline) and positive (histamine acid phosphate) controls, the test was considered as a positive response. Patients with clinical signs of asthma who had a positive skin prick test results were included in the atopic asthma groups. Exclusion criteria for the patient group were as follows: any infection or asthma exacerbation within the previous four weeks; the use of antihistaminic, oral or parenteral steroid within the previous four weeks; any allergic co-morbidity such as allergic rhinitis, and atopic dermatitis; and any systemic disease such as acute or chronic liver disease, etc. The control group consisted of 55 age-matched healthy children (6–14 years). Healthy children were chosen from those referred to the paediatric out-patient clinic for routine pre-op check-ups for their elective surgery (inguinal hernia, tonsillectomy, etc.). Control patients were evaluated with regard to chronic and/or severe infections, autoimmune disorders, and familial and personal history of atopy, and also by laboratory tests. Children were included in the control group if they had no personal and familial history of atopy and no signs of atopic disorder, and if they were negative for skin prick test. As smoking effects oxidative status, patients came from non-smoking households, and the control group was also selected from non-smoking households. Patients taking antioxidant drugs, vitamins, diuretics, hormone replacement therapy and those who smoked were also excluded from study. Blood samples were collected from the patient group for whole blood count, serum IgE level, plasma PON1, TAS and TOS level, and only plasma PON1, TAS and TOS level measurement from the control group. Whole blood count and serum IgE level measurement were analysed via the chemiluminescent enzyme immunoassay method at Immulite 2000 automated analyser (Immulite 2000, DPC, LA, CA., USA).
Evaluation of asthma controlAsthma control was evaluated according to GINA guidelines, by one paediatrician with special interest in Paediatric Allergy (HA) who was blinded to plasma of PON1, TAS and TOS level results.
SamplesBlood samples were obtained following overnight fasting. Blood samples were collected into empty tubes and immediately stored on ice at 4°C. The serum was then separated from the cells by centrifugation at 3000rpm for 10min. Serum samples were stored at −80°C until required for PON1, TAS and TOS analysis.
Paraoxonase activityParaoxonase activities were measured according to a method previously described by Furlong et al.17 Paraoxonase was measured using paraoxon. The rate of paraoxon hydrolysis was measured by monitoring the increase of absorbance at 412nm at 37°C. The amount of generated p-nitrophenol was calculated from the molar absorptivity coefficient at pH 8, which was 17,000M−1cm−1. Paraoxonase activity was expressed as U/L serum (one unit is the number of micromoles of paraoxon hydrolysed per minute).
Measurement of the total anti-oxidant status (TAS) of plasmaSerum samples for measurement of TAS and TOS levels were stored at −80°C until they were used. TAS of serum was determined using an automated method.18 In this method, hydroxyl radical, the most potent biological radical, is produced. The anti-oxidant effect of the sample against the potent free-radical reactions, which are initiated by the hydroxyl radicals produced, is measured. The assay has excellent precision values of lower than 3%. The results are expressed as millimoles of trolox per litre.
Measurement of total oxidative status (TOS) of plasmaTOS of plasma was determined using an automated measurement method, developed by Erel.18 Oxidants present in the sample oxidise the ferrous ion-o-dianisidine complex to ferric ion. The ferric ion makes a coloured complex with xylenol orange in an acidic medium. The colour intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules in the sample. The results are expressed in terms of micromolar hydrogen peroxide equivalent per litre (μmol H2O2 Eq./L).
Ethical approvalThe study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice and was approved by the local Ethics Committee of Vakif Gureba Hospital, and informed consent was obtained from parents or legal guardian of participants.
Statistical analysisSPSS programme (v11.5, SPSS Inc., Chicago, IL, USA) was used for all statistical analysis. Results were presented as mean±SD and percentiles. Differences in parametric and non-parametric values were tested with Student's t-test and Mann Whitney U test, respectively. Categorical variables were compared with Chi-square test. Kruskal–Wallis tests were used for non-parametric analysis. For the comparison of three groups, one-way ANOVA test was used. If the value of P was <0.05 in those tests, post hoc comparison tests were used for the analysis. A receiver operating characteristic curve for the assessment of the predictive value of PON1, TAS, and TOS levels on uncontrolled disease was performed and the area under curve was calculated. Correlation was investigated with Pearson tests for parametric values and Spearman correlation tests for non-parametric values. A value of P<0.05 was considered a statistically significant difference.
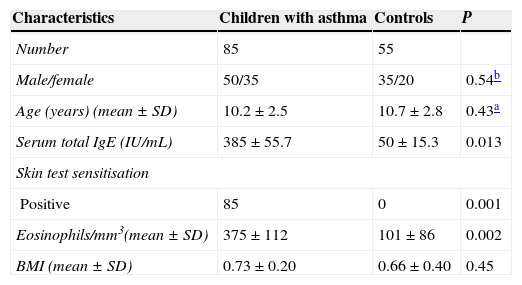
ResultsThe demographic and clinical parameters of the study population are shown in Table 1. There were no differences between the groups with regard to age, sex, and body mass index standard deviation score (BMI SDS). When the asthmatic subjects were compared with the control group, plasma PON1 levels were significantly lower (P<0.05). In addition, TAS and TOS levels in the children with asthma were significantly higher than in the control group (P<0.05) (Table 2).
Some clinical and demographic characteristics of children with asthma and non-allergic controls.
| Characteristics | Children with asthma | Controls | P |
|---|---|---|---|
| Number | 85 | 55 | |
| Male/female | 50/35 | 35/20 | 0.54b |
| Age (years) (mean±SD) | 10.2±2.5 | 10.7±2.8 | 0.43a |
| Serum total IgE (IU/mL) | 385±55.7 | 50±15.3 | 0.013 |
| Skin test sensitisation | |||
| Positive | 85 | 0 | 0.001 |
| Eosinophils/mm3(mean±SD) | 375±112 | 101±86 | 0.002 |
| BMI (mean±SD) | 0.73±0.20 | 0.66±0.40 | 0.45 |
Paraoxonase, total antioxidant status and total oxidative status levels in patients and controls.
| Parameter | Children with asthma (n=85) | Controls (n=55) | Pa |
|---|---|---|---|
| Total antioxidant status (mmol of trolox/L) | 6.9±2.1 | 1.05±0.32 | <0.001 |
| Total oxidative status (μmolH2O2/L) | 12.5±3.4 | 5.5±3.8 | <0.001 |
| Paraoxonase (U/L) | 156.5±55 | 298.6±87.6 | <0.001 |
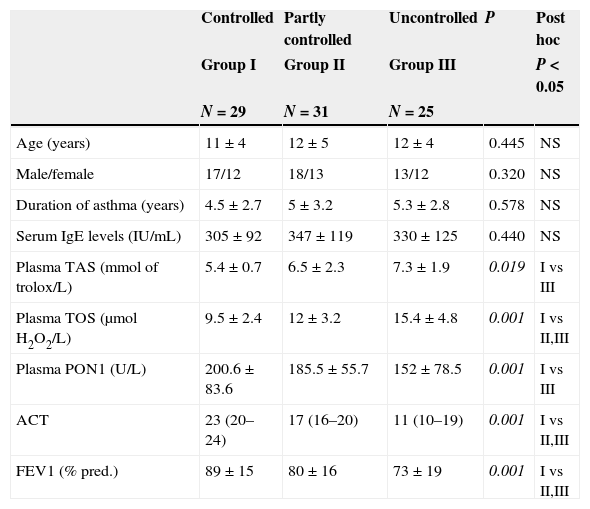
Patients’ demographics and parameters related to asthma controls are presented in Table 3. Patients with partially controlled asthma had higher TAS and TOS and lower PON1 levels compared to those with controlled asthma. Uncontrolled asthma was associated with lower PON1 levels, [r=0.340; P=0.002], higher TAS levels, [r=0.449; P=0.04], and higher TOS levels, [r=0.358; P=0.01], compared to controlled asthma. PON1, TAS, and TOS levels in children with uncontrolled asthma were statistically different from those with controlled asthma, but did not differ from those with partly controlled disease.
Comparison of patients’ demographics and parameters according to the level of asthma control.
| Controlled | Partly controlled | Uncontrolled | P | Post hoc | |
|---|---|---|---|---|---|
| Group I | Group II | Group III | P<0.05 | ||
| N=29 | N=31 | N=25 | |||
| Age (years) | 11±4 | 12±5 | 12±4 | 0.445 | NS |
| Male/female | 17/12 | 18/13 | 13/12 | 0.320 | NS |
| Duration of asthma (years) | 4.5±2.7 | 5±3.2 | 5.3±2.8 | 0.578 | NS |
| Serum IgE levels (IU/mL) | 305±92 | 347±119 | 330±125 | 0.440 | NS |
| Plasma TAS (mmol of trolox/L) | 5.4±0.7 | 6.5±2.3 | 7.3±1.9 | 0.019 | I vs III |
| Plasma TOS (μmolH2O2/L) | 9.5±2.4 | 12±3.2 | 15.4±4.8 | 0.001 | I vs II,III |
| Plasma PON1 (U/L) | 200.6±83.6 | 185.5±55.7 | 152±78.5 | 0.001 | I vs III |
| ACT | 23 (20–24) | 17 (16–20) | 11 (10–19) | 0.001 | I vs II,III |
| FEV1 (% pred.) | 89±15 | 80±16 | 73±19 | 0.001 | I vs II,III |
TAS: total antioxidant status; TOS: total oxidative status; ACT: asthma control test; FEV1: forced expiratory volume in 1s; NS: not significant. The values represent the mean±SD.
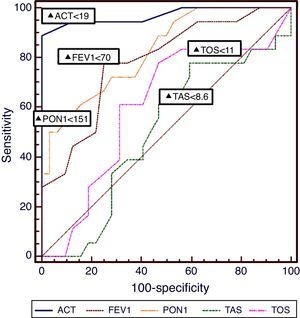
The diagnostic performance of plasma PON1, TAS, TOS levels and ACT and FEV1 results for the identification of patients with uncontrolled asthma is presented in Table 3 and the corresponding ROC curves are shown in Fig. 1. PON1 provided a positive predictive values (PPV) of 0.85at a cut-point of 151U/L, whereas TAS provided a PPV of 0.87 for a cut-off point of <8.6mmol/L, and TOS provided a PPV 0.83 for a cut-off point of ≥11. According to the ROC analyses, PON1 provided relatively higher sensitivity and specificity than TOS. Additionally, FEV1 (% predicted) values ≤70 provide PPV>0.86 for identification of not uncontrolled asthma. According to these ROC results, serum PON1 and FEV1 were both nearly equal for providing PPV. However, the diagnostic performance of these two biomarkers and their combination was inferior to ACT [for a cut-point of <19, (AUC 0.972, 95% CI 0.890–0.985] (Fig. 1). Spearman correlation analysis results pointed out that the two independent variables that cause increased plasma TAS/TOS, and decreased PON1 levels were the attack frequency per year (r=−0.59; P<0.001) and uncontrolled disease (r=−0.89; P=0.001).
DiscussionWe have shown that plasma PON1, TAS, and TOS levels – easily performed blood biomarkers – are related to the level of asthma control in children. We have also observed that the PON1 activity of the asthmatic children was lower than in the control group. Additionally, plasma TAS and TOS levels were significantly higher in asthmatic patient than controls. In contrast with the studies by Ekmekci et al.19 and Górnicka et al.,20 the acquired information about relationship between oxidative stress and paraoxonase activity indicates that lower plasma PON1 level and higher plasma TOS level are closely associated with uncontrolled disease for asthma in children. Also, Can et al.21 observed that asthmatic patients had increased paraoxonase activity after treatment. Evidence of increased paraoxonase activity in asthmatic patients after treatment may indicate that paraoxonase could play a role in asthma. A recent study also confirmed the decreased levels of PON1 associated with plasma TAS and TOS levels inversely.22 The acquired information about the relationship between oxidative stress and paraoxonase indicates that paraoxonase may play a role in the pathogenesis of allergic inflammation. We have shown that serum PON1 values ≥151U/L exclude controlled asthma with an NPV of 0.85. Our study adds to this field as it provides data on the levels of PON1 that may be of clinical importance in the assessment of asthma control in everyday clinical practice.
Elevated levels of reactive oxygen species (ROS) such as hydroxyl radicals, superoxides, and peroxides are capable of producing a variety of pathological changes that are highly relevant in airway mucosa. These include lipid peroxidation, increased airway reactivity, increased airway mucosal sensitivity, and secretions, production of chemoattractant molecules, and increased vascular permeability.23,24 These changes cause airway obstruction, impair ciliary motility, increase the viscosity of airway mucus and damage the airway epithelium, which are crucial for the development of inflammatory airway diseases such as asthma. The evidence for oxidative injury in allergic subjects comes from both systemic and local studies.25,26 Locally, increased production of ROS was shown for eosinophils and macrophage in the airways, and elevated hydrogen peroxide and nitric oxide levels have also been noted in the exhaled breath of patients with asthma.27,28
Increased production of ROS leading to an imbalance between the oxidative forces and the antioxidant defence system favouring an oxidative injury has been implicated in the pathogenesis of asthma. The antioxidant pathways that form the major line of defence against the oxidative insult within the lung can be categorically divided into enzymatic and non-enzymatic system.29 Due to very short half-lives of ROSs it is practically impossible to measure ROSs directly, so indirect measurements of scavenger antioxidant enzymes, such as PON1, are used to evaluate oxidant stress.4,12
TAS and TOS have never been previously evaluated in the assessment of asthma control in children. Excessive production of oxidants occurs spontaneously or after stimulation in blood leukocytes of stable asthmatic patients compared with normal subjects.30 Analysis of TAS and TOS is an automated measurement method, developed by Erel,18 and it is likely to show the systemic production of free radicals. In this method, hydroxyl radical, which is the most potent biological radical, is produced. In our study, TAS and TOS increased together in children with asthma. The increase in plasma TAS and TOS levels in children with asthma in our study supports finding of Cakmak et al.,22 but some of the differences should be mentioned. First, plasma TAS and TOS levels were not evaluated with the disease severity and duration in that study but we found positive correlation between plasma TAS and TOS levels and asthma control test, which may reflect airway inflammation. We did not investigate any relationship between plasma TAS, TOS, and PON1 levels and inflammatory cells in the intravascular compartment including eosinophils, lymphocytes, neutrophils and monocytes because that was not related to our hypothesis. Our study results showed that the performance of TOS was superior to that of TAS in uncontrolled asthmatic children. This acquired information adds to this field as it provides data on the levels of TOS rather than TAS that may be of clinical importance in the assessment of asthma control in everyday clinical practice.
This is the first study to our knowledge that evaluates cut-off points of TAS and TOS for the identification of asthma control. It should be noted that, in a similar manner to PON1, this is at the cost of low NPVs, suggesting that TAS and TOS alone, are not useful for the identification of well-controlled asthma in children. TAS and TOS have been related to levels of oxidative stress in children with asthma,21,22 further supporting a possible role of TAS/TOS levels in confirming true loss of control in children with asthma, as suggested by our data. Inaccurate perception of symptoms is often found in children with asthma.31 Therefore, the need for biomarkers that accurately identify patients with not uncontrolled asthma is imperative, especially in children, and serum PON1, TAS and TOS levels may provide useful information for this age-group. The performance of ACT was superior to that of serum PON1, TAS, and TOS and their combination in our study, yet it was inferior and cannot substitute the clinical evaluation by clinicians with special interest children with asthma.
Despite the invasive nature of this test, it offers some advantages over invasive techniques such as bronchial lavage, induced sputum eosinophil measurement, and bronchial biopsy. Biomarkers of airway inflammation are under evaluation for the guidance of asthma management. Studies have shown that treatment strategies aiming at normalisation of induced sputum eosinophils may reduce exacerbations, without the need for additional anti-inflammatory treatment.32 However, sputum induction is time-consuming and requires a good practical compliance especially in children. The fraction of exhaled nitric oxide (FeNO) has been widely evaluated as a marker of eosinophilic airways inflammation, since it is readily measured and is responsive to changes in inhaled corticosteroid doses.33 However, studies using FeNO guidance for asthma treatment in children have been controversial,34,35 and this may reflect differences in design and the fact that cut-off points used may reflect values from a normal population instead of the population of interest.
In epidemiological studies, pulmonary function tests are considered to be an important diagnostic tool of the presence of airway inflammation and bronchial hyperresponsiveness in asthmatic patients.36 Specific guideline-defined spirometric measures used to classify asthma severity and control, the forced expiratory volume in 1s (FEV1) and the FEV1/forced vital capacity (FEV1/FVC) ratio generally correlate poorly with symptom-based severity in children. A disadvantage to using spirometry in asthma management is that the forced expiratory volume in 1s (FEV1), which is felt to be reproducible and an appropriate measure of airway obstruction, is often normal even in children with symptoms of uncontrolled asthma.37 Therefore, it is difficult to use this variable both in the clinical setting and in epidemiological and clinical trials, in which reliable objective measures of clinical outcomes are needed to provide recommendations for practice. We are intrigued by the detection of TAS/TOS in frozen plasma, suggesting that the specific PON1, TAS/TOS measured in this assay are stable and therefore repeated measurements can be made.
One limitation of our study is the lack of serum HDL and serum protein levels of study group patients. The primary aim of the our study was to evaluate serum PON1, TOS, and TAS levels in children with asthma, and a possible association with each other rather than PON1, HDL, and serum total protein levels. Future studies are highly recommended to extend beyond the scope of our ethical and financial constraints.
In conclusion, we have shown that serum PON1 and TAS/TOS levels may be useful in the identification of children with no uncontrolled asthma. This is the first study that shows negative correlation between the asthma control test and plasma PON1 and TAS/TOS levels. Plasma PON1 and TAS/TOS level may be a useful marker of inflammation in stable asthmatic children and a ≥151mmol/L for PON1 may reflect an uncontrolled disease. A complementary role for two biomarkers was demonstrated in children with asthma. However, their performance was inferior to clinical judgement and may be limited to selected subgroups of asthmatic children. Further longitudinal studies for the prospective evaluation of the treatment response according to PON1, and TAS/TOS and asthma control test, could shed more light on this topic.
Ethical disclosuresPatients’ data protectionThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestThe authors have no financial or personal relationships with other people or organisation that could pose a conflict of interest in connection with the present work.
Institution: Bezmialem Vakif University, Medical Faculty, Istanbul-Turkey. www.bezmialem.edu.tr.