Egg is the food that most often causes allergy in young Spanish children, with an incidence of 2.4–2.6% in the first 2 years of life. The prevalence of sensitisation and allergy to egg is greater in children with allergy to cow's milk and in those suffering atopic dermatitis. The protein component from egg white is the cause of the allergic response in child. The major allergens in egg white are ovomucoid and ovalbumin. Most of the allergic reactions affect the skin, followed by gastrointestinal and respiratory systems. Egg allergy is one of the most common causes of severe anaphylaxis. The diagnosis of egg allergy is based on the existence of a suggestive clinical history, a positive allergy study and the subsequent application of controlled exposure testing, which represents the gold standard for confirming the diagnosis.
The treatment of egg allergy is based on the avoidance of egg protein intake. A subgroup of egg-allergic patients are tolerant to cooked egg. In these cases, only uncooked egg must necessarily be avoided. Maintaining a diet with strict egg avoidance is difficult, and transgressions are relatively common. The patient, family, and school environment should receive education and training in the avoidance of egg and in the management of possible allergic reactions. With an avoidance diet, up to 15–20% of children will remain allergic and the severity of the reactions will increase over the years. In these more severe cases of egg-allergy, it becomes more difficult to adhere to the avoidance diet over the years, with a significant decrease in patient quality of life.
Oral tolerance induction can be regarded as a therapeutic option for IgE-mediated egg allergy. The anti-IgE, omalizumab, might become another genuine therapeutic option for food allergy, not only to prevent allergic reactions after a contact with egg, but also as a complementary treatment to oral tolerance induction for egg allergy, with the purpose of reducing adverse reactions.
The administration of influenza vaccine to children with egg allergy is safe in children that do not manifest severe reactions after egg intake, and in children who tolerate cooked egg. The triple viral vaccine (MMR) can be given to egg-allergic children in their usual vaccination centre, with no added risk. Different medicinal products can be formulated with egg proteins, and therefore should be avoided in children with egg allergy.
According to the classification proposed by the European Academy of Allergy and Clinical Immunology (EAACI),1 chicken egg allergy is an adverse reaction with an underlying immunological pathogenic mechanism produced by the intake or contact with egg and its proteins.
Although other possible immunological mechanisms may be implicated, in the case of egg allergy the currently only well known mechanism corresponds to an IgE-mediated type I immediate hypersensitivity reaction.
Eggs, typically represented in our setting by chicken eggs, represent an important source of proteins, and form one of the basic elements in our diet from the first year of life.
Their high protein content, introduction in the diet from the first year of life, and their widespread consumption define eggs as the food that most often causes allergy in young children in Spain.2
Epidemiological dataEgg is the leading cause of food allergy in children.2–4
Such allergy tends to manifest under 2 years of age, and 50% of the patients are able to reach tolerance by 3–4 years of age, versus 66–74% by 5 years of age.5–7
The literature reports a 1.6% incidence of symptomatic egg allergy in the first year of life, and a cumulative incidence of 2.4–2.6% in the first 2 years.8,9
In Spain, an observational study has been carried out, known as the Alergológica survey,10 involving 4991 patients visiting the allergologist. Food allergy was diagnosed in 369 patients (7.4%). In this group, egg allergy accounted for 16% of the cases of food allergy, and egg was seen to be the fourth ranking causal food by ranking of frequency in the general population, and the leading cause in children less than 5 years of age. In this latter subgroup, egg allergy accounted for 78.9% of all cases of food allergy, and was the main sensitiser along with cow's milk. In patients with atopic dermatitis and digestive symptoms, milk and egg were the most frequent causal allergens. In individuals under 15 years of age, the frequency of egg allergy was 20%, and eggs, milk, and nuts were found to be the leading cause of food allergy in this age group.
Most sensitisations to egg protein (76%) occur before 5 years of age, 12% between 5 and 10 years of age, and 12% between 10 and 15 years of age.
In a group of 355 children diagnosed with food allergy in Spain,2 the prevalence of allergy to egg proteins was 20.1% – this figure is similar to that recorded in the Alergológica survey. A little over one-half of the patients (56.5%) developed the symptoms between 6 and 12 months of age, and 97% of the subjects manifested in the first 2 years of life. Only 16% of children with egg allergy had other associated food allergies (three or more).
The prevalence of sensitisation and allergy to egg is greater in children with allergy to cow's milk and in those suffering atopic dermatitis.
In infants with allergy to cow's milk, sensitisation to egg has been documented in 30–67% of the cases before its introduction in the diet, and positive provocation tests have been recorded in 36%.11–13
In infants with atopic dermatitis, sensitisation to egg protein has been observed in 61% of patients before its introduction in the diet, and positive provocation tests have been recorded in 27–67%.14–16
AetiologyEgg contains abundant proteins of high biological value, and is widely consumed throughout the world.
Egg allergy occurs when its proteins, or allergens, are recognised by the host as foreign, and the immune system consequently develops a rejection response to them.
Both components of egg, the yolk and egg white, can cause allergic sensitisation, although, particularly in children, the protein component in egg white is the cause of the allergic response. Crossed immunoelectrophoresis has identified at least 24 different proteins in egg white, but only some of them cause allergic reactions. Exceptionally, some patients can develop allergy to proteins contained in egg yolk, while preserving tolerance to the egg white proteins.
Pathogenic mechanismEgg proteins usually come into contact with the host immune system through the digestive tract. From the first stages of life the infant can come into contact with food proteins through breast milk,17 via the inhalatory route, or skin contact. Occasionally, first contact can occur during foetal development through the placenta.
The body has a series of physiological barriers that protect it from foreign antigens. In the digestive system, these barriers comprise two groups of elements: (1) non-immunological (gastric acid, pancreatic enzymes, intestinal enzymes, mucus, the membrane of the microvilli, the mucosal layer and intestinal peristalsis), and (2) immunological (IgA, IgE, IgM, IgG, lymphocytes and macrophages, Peyer's patches, intestinal secretory IgA and secretory IgA in breast milk).
When the composition of the diet is modified, as for example when new foods are introduced or when the infant is weaned from breastfeeding, complex physiological changes take place. This situation can have a profound impact on the immune response, not only because the antigens found in the lumen are different, but also because of the changes in ingestion and digestion. The mucus and proteolytic enzymes contained in the digestive secretions, bowel motility, the rate of absorption and intestinal transit, all influence the amount of antigen present in a given segment of the intestine.
Most proteins in the diet are broken down to amino acids through the action of proteolytic enzymes during digestion, although 2% of the ingested proteins are absorbed as immunologically recognisable peptides. These proteic structures can be linear epitopes or conformational epitopes with a three-dimensional structure. The immune response of the gastrointestinal mucosa is characterised by an intricate balance between host defense and immune regulation. Normally, when the immune system recognises food proteins as being foreign to the host, immunoregulatory mechanisms are established that lead to the acquisition of tolerance. Alterations in these regulatory mechanisms alter the induction of tolerance, resulting in food allergy. Different factors, including age, the genetic susceptibility and commensal intestinal flora of the individual, antigen exposure route and solubility, the presence of other proteins, lipids and vitamins, and especially the type of antigen-presenting cells (APCs), can condition the induction of tolerance.18,19 The mechanisms underlying the induction of oral tolerance include anergy/deletion or active suppression mediated by regulatory T lymphocytes (Treg), which play a key role in the development of peripheral tolerance. These cells suppress the immune response by inhibiting the generation of effector T cells in lymphoid tissue and in the target organs through the production of cytokines.20–22 Furthermore, transcription factor FOXP3 is an essential regulating element of the Treg cell line.23 Food allergy results from failure to establish tolerance or from the loss of acquired tolerance.
IgE-mediated reactionsImmunoglobulin E-mediated allergic reactions to egg are the best characterised allergic reactions to this type of food. Failure to develop oral tolerance results in an excessive production of specific IgE antibodies. These antibodies bind to the high affinity receptors (FcєRI) on mast cells and basophils, and to the low affinity receptors Fc¿RII (CD23) on macrophages, monocytes, lymphocytes and platelets. When the egg allergens penetrate the mucosal barriers and bind to the IgE of mast cells and basophils, these cells release mediators that cause vasodilatation, smooth muscle contraction and mucosal secretion, giving rise to the typical symptoms of immediate hypersensitivity. The activated mast cells can also release cytokines that contribute to the delayed phase of the response. In the first 4–8h, neutrophils and eosinophils invade the site of the response and release different mediators such as platelet activating factor (PAF), peroxidases, and eosinophil cationic protein and major basic protein.24 Finally, in the subsequent 24–48h, chronic inflammation is observed of the affected areas, with the infiltration of lymphocytes and monocytes. These cells can release histamine releasing factor (HRF), which may be responsible for cutaneous and bronchial hyperresponsiveness.
Non-IgE mediated hypersensitivityIn some adverse reactions an immunological mechanism is believed to be implicated, although the underlying pathogenic factors have not been well defined.
At present, type II and III hypersensitivity reactions are not believed to play an important role in egg allergy. However, cell mediated type IV hypersensitivity appears to be involved in those reactions that start several hours after exposure to the antigen. This type of hypersensitivity is found in conditions such as eosinophilic gastroenteritis and oesophagitis, and in atopic dermatitis.
Egg allergensOf the 24 different proteins known, only some are allergenic and can produce IgE-mediated allergic responses. In this context, ovalbumin (Gal d 2) constitutes 54% of the proteins of egg white, while ovotransferrin or conalbumin (Gal d 3) represents 12%, ovomucoid (Gal d 1) 11%, lysozyme (Gal d 4) 3.5%, ovomucin 1.5%, and other less well known proteins (including avidin, ovoinhibitor, flavoproteins and catalase) constitute over 18%.
The major allergens in egg white are ovomucoid and ovalbumin. The former maintains its immunogenicity after 20min of boiling. As a result of its resistance to heat and enzymatic digestion, and its physical characteristics, ovomucoid is the most important egg white protein capable of causing allergic reactions. Sensitisation to ovomucoid is a marker of persistent egg allergy and of the absence of tolerance to boiled egg. Ovalbumin is the most abundant protein in egg white, but is more sensitive to heat, and loses its allergenic capacity after exposure to high temperatures for shorter periods of cooking time. Patients with sensitisation only to ovalbumin tolerate well-cooked egg.25–27
Since conalbumin (Gal d3) and lysozyme (Gal d4) are more thermolabile, they are weaker antigens but nevertheless can also produce allergic reactions when consumed more or less raw. Lysozyme-specific IgE sensitisation can be responsible for allergic reactions to drugs or foods manufactured with this protein. Less commonly, ovotransferrin can produce allergic reactions in patients allergic to egg who receive oral iron therapy with products based on this protein.
Cross-reactions are observed between egg proteins from different avian species (chicken, turkey, duck, seagull). This means that patients with allergic reactions to chicken egg usually develop clinical manifestations after consuming or coming into contact with eggs from other avian species.28
The yolk contains three main protein fractions capable of binding IgE, and which are identified as granules, livetins and low-density lipoproteins. Among the lipoproteins, apovitelin I and apovitelin VI are of importance. The apovitelins would act as major antigens, and α-livetin or chicken serum albumin (Gal d5) plays an important aetiological role in so-called bird-egg syndrome. Alpha-livetin or serum albumin is present in chicken feathers, meat and eggs. This explains the symptoms of bird-egg syndrome, where the patient develops clinical manifestations upon inhaling particles from feathers and on consuming chicken meat or eggs. Chicken serum albumin is thermolabile, and its clinical reactivity in the presence of specific IgE decreases 88% after heating to 90°C during 30min. In bird-egg syndrome, initial sensitisation may be induced by aeroallergens from other bird species such as parrots, canaries or parakeets. The presence of airborne avian serum albumin has been demonstrated in the domestic environment of patients with bird-egg syndrome.29–31
Individuals allergic to egg white are rarely sensitised to the meat of avian species, and only occasionally yield positive allergic test results. They usually show good tolerance of these foods.
Clinical manifestationsThe clinical symptoms of egg allergy develop in the first 2 years of life, coinciding with the introduction of egg white in the diet, and their onset beyond 2 years of age is rare.7
The appearance of the clinical manifestations is directly related to the age of the individual at the time of introduction of egg in the diet, as well as to the way in which the egg is prepared for consumption: cooked, raw, yolk, egg white, etc.
Allergic reactions to egg most often constitute IgE mediated reactions, although non-IgE mediated reactions have also been described.32–34
Most of the reactions (up to 90%) affect the skin, followed by the gastrointestinal (up to 60%) and respiratory system (up to 40%).35 Egg allergy is one of the most common causes of severe anaphylaxis.36 The skin reactions can comprise erythema, pruritus, urticaria, angio-oedema or a combination of these manifestations. The digestive reactions typically affect the upper gastrointestinal tract and are characterised by nausea, vomiting, colic type abdominal pain and reflux in the first hour after feeding that can manifest as irritability in nursing infants. Respiratory symptoms are less frequent than digestive or cutaneous manifestations, and consist of rhinorrhoea, wheezing or dyspnoea. Hyperaemic conjunctival manifestations can also be observed.37 Another clinical manifestation of egg allergy is patient rejection of the food – a fact that can delay the diagnosis for years, since the patient remains unexposed to sufficient amounts of the allergen to allow clear manifestation of the symptoms.
The most common scenario is a reaction following the consumption of whole egg, after having previously tolerated the usually cooked egg yolk.
Non-IgE mediated egg allergy is much less frequent than IgE mediated allergy. Egg may be one of the food allergens implicated in eosinophilic oesophagitis, gastroenterocolitis and proctocolitis, although the frequency with which egg causes these conditions is low.33,38 In these cases the presenting clinical manifestations depend on the affected anatomical location. Thus, in the case of enteritis or enterocolitis, the clinical manifestations usually start in the form of later onset vomiting (2–3h after ingestion), with severe involvement of the general condition of the patient, prostration, paleness usually without hypotension (though hypotension may also be noted), followed or not by diarrhoea. In turn, proctocolitis manifests as stools containing red blood with mucus in nursing infants that are otherwise in excellent general condition and are breastfed. These stools may worsen hours after the mother has consumed egg. Allergic proctocolitis is more often produced by cow's milk, but egg is also a potential cause.39 Eosinophilic oesophagitis is characterised by vomiting, abdominal pain and dysphagia. Very young infants may also present loss of appetite, weight loss or food rejection (aversion), while older children can suffer stomach discomfort, food impaction, nausea or retrosternal pain. The symptomatology in some cases is similar to that of gastro-oesophageal reflux.
Separate consideration is required for atopic dermatitis, in which positive IgE-specific and skin tests for egg allergens often coexist in the absence of clear clinical antecedents. In many cases egg has not yet been introduced in the diet, and the test results only reflect sensitisation.40
If egg has already been introduced in the diet, a test period involving total egg suppression during 2–4 weeks may be required, followed by the reintroduction of egg. A possible relationship between egg consumption and the exacerbation or chronification of dermatitis can be observed in a few cases, and after a period of exclusion from the diet, egg reintroduction may give rise to immediate skin or digestive manifestations.41–43
DiagnosisAs with all food allergies, the diagnosis of egg allergy is based on the following:
- •
The existence of a suggestive clinical history.
- •
A positive allergy study.
- •
The subsequent performance or not (depending on the circumstances) of controlled diagnostic exposure testing, which represents the gold standard for confirming the diagnosis.
The clinical diagnosis requires a detailed anamnesis, which must include the following:
Regarding the food- •
Age at the time of introduction of whole egg and of yolk and egg white separately.
- •
Age at the time of the first reaction, specifying whether it involved whole egg or not, and whether it corresponded to apparent first exposure to egg, or whether the infant had already been consuming eggs for some time.
- •
The symptoms usually manifested with whole egg. It is not uncommon to observe prior tolerance to egg yolk, which in our setting is usually introduced in the diet at an earlier stage, separate from egg white, and almost always cooked.
- •
Tolerance or intolerance of the different cooking presentations (cooked, raw, omelette, semi-raw, etc.), along with the assessment of subsequent tolerance after the clinical manifestations leading to consultation.
- •
The amount of food producing the reaction is indicative of the severity of the allergy.
- •
The symptoms are usually caused by oral intake of the food or by its presence in other foods in the form of a hidden allergen, although they can also be produced by direct or indirect skin contact with egg (kisses, caresses, playing, and exposure to volatile particles of beaten egg). This aspect should always be considered.
- •
A precise description of the symptoms is required.
- •
Latency between food intake and appearance of the symptoms. In this context, immediate symptoms onset, or their appearance within no more than 60min after ingestion, is suggestive of IgE-mediated allergy.
- •
Treatment required and time to resolution: these aspects are indirectly indicative of the severity of the condition. On the other hand, persistence of the symptoms for more than 12h or their subsequent exacerbation should lead us to consider other disease processes.
- •
Number of episodes and their description. Several episodes clearly related to the consumption of egg represent the strongest diagnostic evidence.
- •
Time elapsed from the last symptoms experienced by the patient. Recent symptoms are suggestive of current allergy, while re-evaluation is indicated if the last symptoms were observed a long time ago.
- •
In infancy, the diagnosis of egg allergy should be re-evaluated on a regular basis, since sensitisation in most cases is only temporary.
- •
Skin tests or serum IgE determinations often detect sensitisation to egg in patients with allergy to other foods or in patients with atopic dermatitis, even before first ingestion takes place. In such cases sensitisation, not allergy, will be assumed as long as no symptoms are observed.16,44
- •
Data referring to the following must be collected in all cases:
- ∘
Family history of atopy
- ∘
Allergy to other foods
- ∘
Asthma or wheezing
- ∘
Atopic dermatitis
- ∘
The anamnesis should be completed with a detailed physical examination, placing special emphasis on the presence of skin manifestations consistent with atopic dermatitis, since the latter is often associated to egg allergy.
Clinical differential diagnosisCutaneous symptoms such as erythema, urticaria or oedema, as well as gastrointestinal symptoms in the form of vomiting or diarrhoea, are to be distinguished from those caused by viral diseases, which are very common in the first stages of life.
Referring to purely gastrointestinal manifestations, we also must assess the possibility of bacterial infection secondary to food contamination.
The following elements clearly speak in favour of allergy:
- •
A short latency between egg intake and appearance of the symptoms.
- •
The absence of fever and of contexts suggestive of infection.
- •
Disappearance of the clinical manifestations within hours, either with or without treatment.
- •
If the triggering factor is allergenic, the patient will remain free of symptoms unless exposure to the food is repeated.
From the practical perspective, a negative result for ovomucoid (OVM Gal d1) in both skin and serum IgE testing is a good predictor of tolerance of cooked egg – this in turn implying a radical change in the dietetic treatment indications.
The allergens to test for are ovomucoid (OVM Gal d1), ovalbumin (OVA Gal d2), egg white and yolk, and this can be complemented by testing for other minority allergens such as conalbumin and lysozyme. Alpha-livetin or chicken serum albumin (Gal d5) is implicated in bird-egg syndrome.
Intraepidermal testing – prick testThe antigens used for skin tests can be commercial extracts of whole egg, egg white or yolk separately, and especially the allergens that are most relevant from the practical tolerance perspective, i.e., OVA and OVM.
The commercial allergenic extracts of egg offer high diagnostic sensitivity. Use is made of glycerinated extracts at a concentration of 10mg/ml for egg white and yolk, and of 1mg/ml for OVA and OVM. The test is considered positive when a wheal measuring ≥3mm in size is produced. Use of the fresh food offers few additional advantages, since the sensitivity of the commercial extracts is high.45 Only in negative test cases with suggestive clinical manifestations does the use of the fresh food increase the sensitivity of the skin test and improve correlation with the provocation test.
Skin tests performed with the prick technique have high but variable sensitivity, and lesser specificity. The positive predictive value is low, and the negative predictive value is very high; as a result, negativity of the skin tests using an adequate extract practically rules out egg allergy.46,47
Serum specific IgEThe determination of serum specific IgE poses the same interpretation problems as in the case of the skin tests. A negative determination for OVA and OVM has a high negative predictive value but does not exclude clinical reactivity. The positive predictive value in turn varies according to the prevalence of the disease and the type of associated pathology (atopic dermatitis).48,49
According to the conclusions of the study published by Boyano et al., in infants under 2 years of age with cutaneous, digestive and/or respiratory manifestations starting in the first 2h after egg intake and with a positive prick test for egg white, egg white serum specific IgE values of ≥0.35KU/l predict clinical reactivity in over 90% of the cases. Therefore, when all these criteria are met, the diagnosis can be taken to be firm, and diagnostic exposure testing does not prove essential.50
Controlled oral exposure/provocation testControlled oral exposure/provocation testing may be indicated for confirming the diagnosis, and especially for assessing evolution towards tolerance.
The methods and conditions described in the position documents of the European Academy of Allergology and Clinical Immunology and of the Spanish Society of Allergy and Clinical Immunology51,52 are the ones we also recommend.
An informed consent document must be signed in all cases, to be included in the patient history.
The patient must be free of symptoms, without antihistaminic medication, for at least 3 days, and with an empty stomach (a light breakfast can be accepted 1–2h before in small infants). The patients must remain in a comfortable environment where they can be observed by trained staff in the early detection and treatment of symptoms.
IndicationsProvocation testing is indicated in all patients with suspected allergy to egg (on the basis of the clinical history, positive skin tests, positive IgE), in which diagnostic confirmation is considered necessary. It is also indicated in those cases with a time from the last clinical episode of not less than 6 months in the second year of life and no less than 1 year in older patients, with the goal of assessing evolution towards tolerance. The mentioned IgE cut-offs can be used as an orientation, but always taking into account that they are group predictive, not predictive of individual cases, and that the other above-mentioned diagnostic tests must be used for confirmation purposes. In patients sensitised to egg without previous exposure to the food, controlled exposure should be made53 before introducing egg in the diet.
ContraindicationsSevere anaphylaxis to egg allergy is not an absolute contraindication, but requires more careful revision of the interval from the first episode, in order to extend it, and the assessment of skin tests, variations in IgE values, and other diagnostic methods.
The existence of a recent episode (3–6 months), with a clear and unequivocal history, and with positive prick and IgE tests, should be regarded as a contraindication.
Controlled oral exposure/provocation test technique (Table 1)- (a)
Provocation testing is carried out with egg white. Only in cases where yolk reactivity is suspected do we perform testing with egg yolk and egg white separately and on different days, beginning with yolk. The usual situation in children is allergy to egg white, but there may be patients with allergy to yolk and good tolerance of egg white.
- (b)
Careful separation of egg yolk and egg white must be made, washing the cooked yolk. In the case of raw yolk, the latter is to be extracted by puncture without rupturing the vitelline membrane, in order to avoid contamination with egg white.
- (c)
Testing is started with cooked egg white, and only if this proves negative do we use raw egg white or, better still, pasteurised egg white in order to avoid bacterial contamination.54 Some patients can tolerate thoroughly cooked egg but suffer even serious symptoms with raw egg.55 The maximum starting dose in provocation testing has been defined as 100mg.56 Nevertheless, the starting dose should be individualised for each patient.
- (d)
In order to guarantee good tolerance, the total dose ingested by the patient should be equivalent to a whole raw egg. It can only be affirmed that the patient is fully tolerant if good tolerance to raw egg is confirmed – this culinary form of the food is very common in Spain (sauces, ice cream, smoothies, toppings, etc.).
- (e)
In small infants and in patients with objective clinical manifestations, open provocation testing can be used for practical reasons. In patients with clinical manifestations that prove doubtful or are difficult to evaluate, such as abdominal pain, simple or double-blind testing is used. The latter proves essential in the research setting.
Table 1.Egg exposure/provocation test.
Sequence 1. Cooked egg white 2. Pasteurised egg white Dose 1. Cooked egg white (weight 40g): a. 2.5g (1/16) b. 5g (1/8) c. 10g (1/4) d. 20g (1/2) (approximately 4g accumulated proteins) 2. Pasteurised egg white ½ egg white=15ml=1650mg a. 1ml=110mg b. 2ml=220mg c. 4ml=440mg d. 8ml=880mg e. 16ml=1760mg Time intervals between doses: doses administered every 30–60min, with possible variations according to the latency period between egg administration and the onset of symptoms as documented in the clinical history.
Observation period: monitoring is advised for at least 2h after administration of the last dose.
If we wish to evaluate the clinical relevance of sensitisation to egg in a patient who is consuming egg with good tolerance and presents atopic dermatitis, we must prescribe an egg exclusion diet for at least two weeks,57 followed by a double-blind provocation test with an interval of at least 48h between placebo and the active ingredient, in order to assess late responses. Only if we observe improvement of the dermatitis with the exclusion diet, and its exacerbation with provocation testing, can we accept that egg may influence the course of the dermatitis. However, it must be noted that an exclusion diet applied for a sufficiently long period of time can lead to loss of egg tolerance and thus may give rise to acute symptoms in the form of urticaria, vomiting or respiratory manifestations characteristic of immediate reactions, upon performing the provocation test.
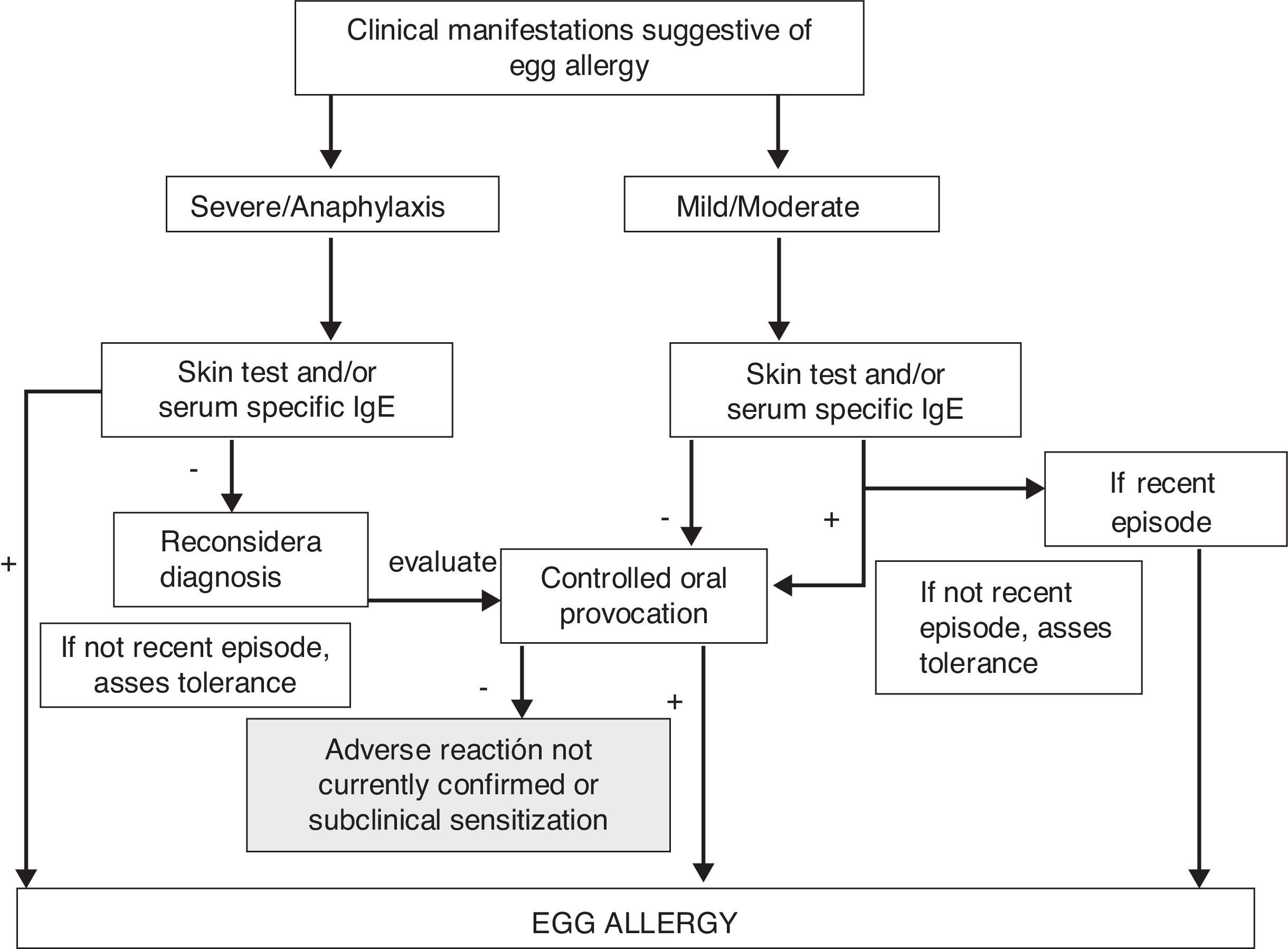
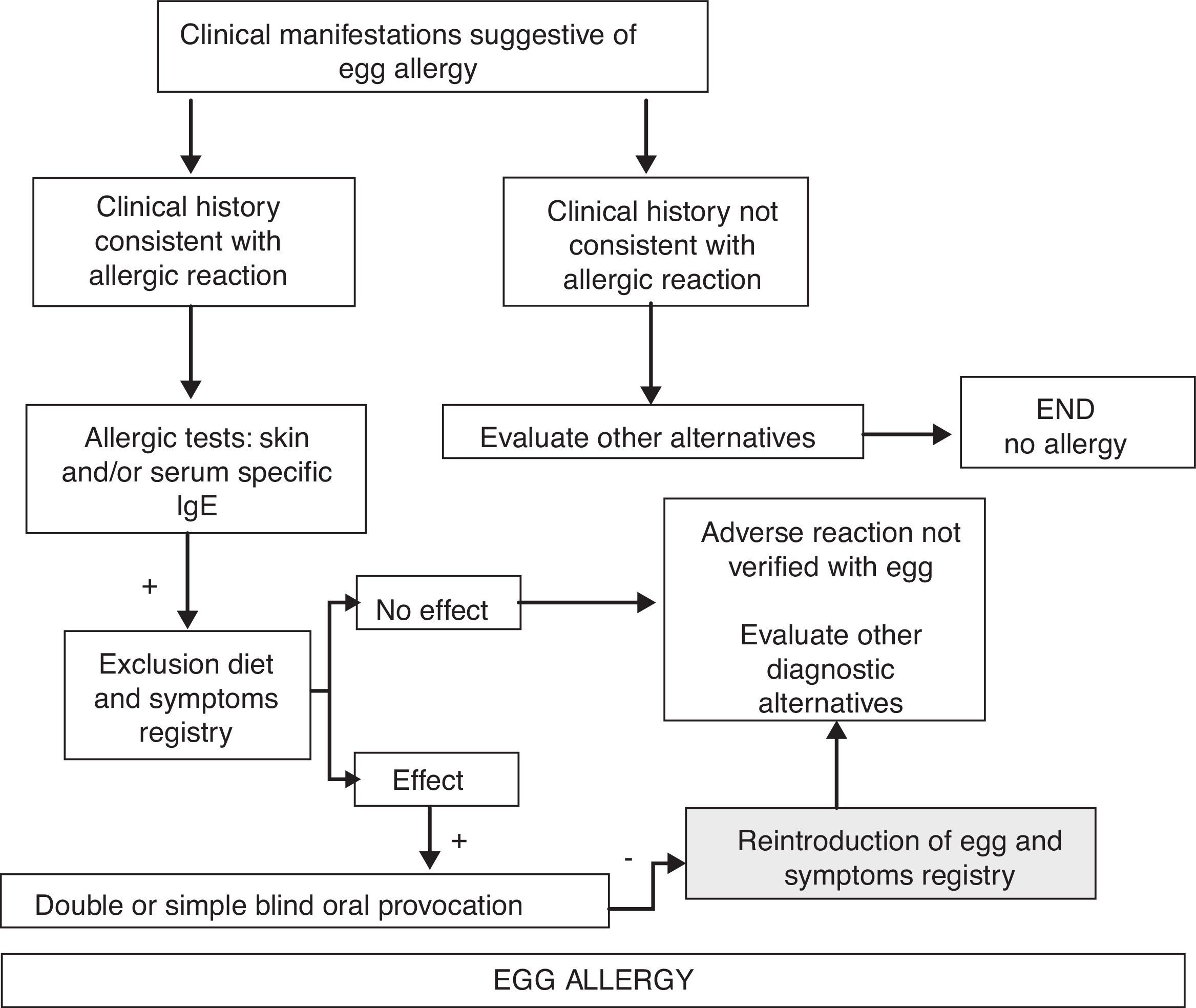
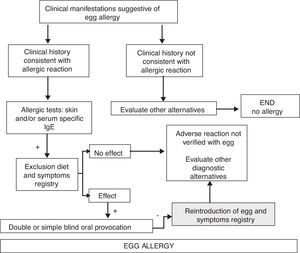
Diagnostic algorithm of adverse reactions with acute symptoms in Fig. 1, and with chronic symptoms in Fig. 2.
Diagnostic algorithm of egg allergy. Acute symptoms.
Diagnostic algorithm of egg allergy. Chronic symptoms.
In recent years, the use of other clinical techniques has been contemplated as a supplement to the already mentioned methods. In this context, labial food challenge (LFC)58–60 and the rubbing test or skin application food test (SAFT) are useful, offer rapid results, are safe, and offer a high negative predictive value61 compared with oral provocation testing in highly sensitised patients or individuals with serious clinical manifestations.
TreatmentAvoidance diet and symptomatic treatmentThe treatment of egg allergy is based on the avoidance of egg protein intake. In the case of children with confirmed tolerance of cooked egg, only uncooked egg is to be avoided (fresh mayonnaise, toppings, omelette, ice cream). Maintaining a strict egg avoidance diet is not easy, and transgressions are relatively frequent and can prove serious – in most cases being triggered in the context of normal daily life situations.62 The lowest observed egg protein dose capable of triggering a reaction may be as low as 2μg in one out of every one million patients with egg allergy, and 3.4mg in one out of every one hundred cases.63,64
There is a direct relationship between the amount of egg consumed by the mother and the concentrations of ovalbumin in breast milk.65,66 In infants sensitisd to egg and who are being breastfed, the need for an egg exclusion diet in the mother should be considered based on the observation of the appearance of clinical symptoms in the infant after breastfeeding.
The patient, family and school environment should receive education and training in the avoidance of egg and in the management of the possible adverse reactions. Vigilance should be maximised, carefully checking the labelling of processed foods. In this context, current legislation, as reflected by Spanish Royal Decree (RD) 1245/2008, of 18 July, and regulation (EU) 1169/2011 of the European Parliament and of the Council, of 25 October 2011, which publish the list of allergenic ingredients of obligate declaration, requires such labelling to identify those products which contain egg or its products. Many processed products and dishes containing egg are sold in fast food establishments, bakeries and restaurants, where the ingredients are sometimes not easy to identify, and where egg cross-contamination is much more likely. Likewise, some proteins of egg origin such as lysozyme are used as bactericidal agents in certain foods. Other products such as sweets, cold meats, pasta, beer and wine can also contain egg proteins, in the same way as certain cosmetic products. Websites are available where information can be obtained on the presence of egg in different foods and other commonly consumed products (for example: www.seicap.is/familiares.asp).
Cross-reactivity exists between chicken egg protein and the eggs of other avian species; as a result, the consumption of such eggs should also be avoided. There is usually no clinical cross-reactivity between chicken eggs and meat,67 and in most cases chicken meat does not have to be excluded from the diet. On the other hand, there have been reports of allergy to poultry without concomitant allergy to egg.68 In patients with allergy to feathers, egg tolerance should be checked and, conversely, patients with egg allergy should be careful with exposure to avian antigens.69,70
In the case of accidental ingestion, medical treatment will depend on the severity of the reaction.71 The description of such treatment extends beyond the scope of this review, however.
The exclusion of egg from the diet does not entail major nutritional problems, except in those patients where food allergy is of a multiple nature, in which case evaluation by the paediatric nutritionist may be necessary to assess the nutritional suitability of the diet with a view to incorporating the necessary supplements.72
The continued consumption of cooked egg can favour tolerance.73 It has been shown that after 3, 6 and 12 months of continued consumption of cooked egg products, the skin test wheal diameters decrease, along with the ovalbumin specific IgE levels, while the IgG4 titers to ovalbumin and ovomucoid increase.74 These studies were not of a controlled nature, however, and randomised trials are therefore needed to confirm the data obtained.75
A recent publication recommends that in the case of patients presenting egg allergy with mild symptoms and without asthma, cooked egg should be reintroduced at 2–3 years of age in the home, and once tolerance has been achieved, this should be followed by the incorporation of less thoroughly cooked egg.76,77 The position of the Food Allergy Committee of the SEICAP is that until clearly identified and contrasted markers become available, ensuring that the introduction of egg in an allergic patient is safe, such procedures should always be carried out in a prepared setting, in order to be able to treat any adverse reactions that may develop.
It cannot be affirmed that the child has acquired tolerance until egg intake no longer produces symptoms, independently of the way in which it is prepared for consumption.
Chromone and antihistamine treatments have been used on a prophylactic basis to avoid clinical manifestations in cases of dietetic transgression, although with variable efficacy, and in no case have such treatments been shown to offer better results than the strict avoidance of egg intake.78,79
Oral tolerance induction (OTI)Even with an avoidance diet, up to 15–20% of the children remain allergic, and dietetic transgressions may occur that often prove serious. The severity of the reactions usually increases over the years, and it becomes more difficult to adhere to the avoidance diet – this situation resulting in school and social problems, with a significant decrease in patient quality of life.
In 1908 the first description was published of a child with egg allergy (anaphylaxis) satisfactorily treated with “oral immunotherapy” (oral tolerance induction, OTI).80 At present, this treatment can be regarded as a management option for IgE mediated egg allergy.81 Basically, OTI consists of the intake of progressively increasing amounts of egg protein, involving very diverse administration protocols, until a non-restricted or free diet is achieved, if possible. OTI must always be provided in a setting prepared to detect and treat any adverse reactions that might arise. The parents and the patient likewise must be trained to detect and deal with these reactions, fundamentally as refers to the use of adrenalin self-injectors. If the patient has asthma, it is essential for the disease to be adequately controlled. The way in which the egg is administered differs according to the protocol employed, ranging from the use of egg white or whole egg, either lyophilised or pasteurised.
The studies found in the literature suggest that most patients with food allergy can be desensitised by means of this process with lesser efficacy than in the case of allergy to cow's milk.82 Adverse reactions are frequent during the desensitisation period; all such reactions are controllable, however, and the literature documents no case of death during OTI.83
One of the first articles on OTI was published by Patriarca84 in the year 2003, involving 15 children with egg allergy, and resulting in a success rate of 83% and a 51% incidence of adverse reactions. Staden,85 in the first randomised clinical study with one group of patients assigned to OTI and another assigned to a strict avoidance diet, involving a series of 11 children with egg allergy and 14 with allergy to cow's milk, reported four response profiles: (a) 36% presenting permanent tolerance despite a period of diet after OTI with posterior reintroduction of the food (tolerant subjects); (b) 12% presenting tolerance with regular intake (desensitised); (c) 16% presenting partial response in which the dose required to trigger symptoms was increased; and (d) 36% presenting failure due to severe reactions in response to very small doses.
In 2007, Morisset86 conducted a randomised clinical study involving 90 children with egg allergy and 57 with allergy to cow's milk. The success rate was 69%, but there were no significant differences between the avoidance group and the treatment group. Buchanan,87 in seven children without anaphylaxis, reported a success rate of 57%. In Spain, Garcia-Rodriguez88 administered a rapid regimen in 23 children, with a duration of 5 days, yielding a success rate of 86.9% and an adverse reactions rate of 78.3%. In this series the patients were considered to be desensitised when they were able to tolerate only 8ml of pasteurised raw egg (1/4 of an egg); as a result, adverse reactions could have developed with higher intakes. In another series of seven patients with severe egg allergy,89 the starting phase was carried out in 18 days until tolerance of 1g of egg white was achieved, with a maintenance phase of 9–12 months. The resulting success rate was 100%, but the adverse reactions rate during treatment was likewise 100%.
Most studies involving OTI in patients with egg allergy have reported satisfactory results, since even in those cases where the final dose cannot be reached, the dosage required to trigger reactions is effectively increased versus the baseline situation – thereby contributing to protecting the patient against allergic accidents resulting from error in the avoidance diet. The question of whether the tolerance achieved is permanent or represents desensitisation in which the sustained and constant intake of egg is what prevents the triggering of symptoms, remains to be established.90 The appropriate patient age for starting this type of treatment also remains to be determined. However, in view of the tendency in OTI among patients with allergy to cow's milk, comprising introduction of the technique at increasingly younger ages with better results and fewer side effects, it may be assumed that the tendency in application to egg allergy will be to start this therapy as early as possible. The adverse effects are frequent and unpredictable, and almost always occur in the context of dose or concentration escalation which is usually carried out in the hospital setting, but which is also found in the home where the patients are taking the previously tolerated dose.91 Many factors can act as triggering elements–the most important being physical exercise after intake of the food, intercurrent processes, or the administration of anti-inflammatory drugs. In some cases, however, no apparent triggering cause can be identified.92
No studies have been made warranting the usefulness of OTI in non-IgE mediated allergic reactions.
Other alternative methods for achieving tolerance are the sublingual93–95 and epicutaneous routes,96 which may be reserved for the more serious cases, or for patients in which OTI has failed.
Anti-IgE (omalizumab)Omalizumab is a humanised monoclonal antibody that binds to circulating IgE, forming macrocomplexes that prevent the immunoglobulin from binding to the IgE receptors on mast cells, basophils, dendritic cells and lymphocytes – thereby reducing the release of mediators that are responsible for the clinical manifestations of allergic reactions. In the management of food allergy, this treatment has been used in two situations:
Prevention of allergic reactions after contact with egg. Most of the studies to date have been made with peanut and milk, with the observation of an increase in the food dose needed in order to trigger symptoms.97 Some of these publications have recorded the same effects in cases of severe egg allergy.
Treatment added to SOTI for egg allergy, with the purpose of reducing adverse reactions.
In some patients SOTI for egg allergy can pose problems due to the appearance of serious reactions even with minimum egg doses, or because the intensity and frequency of the reactions may cause the patient to abandon the treatment. The idea underlying omalizumab therapy would be that the resulting decrease in circulating IgE titers would lead to down-regulation of the expression of high-affinity IgE receptors and probably also to a decrease in IgE production as such – thereby resulting in fewer adverse effects during SOTI, which possibly could even be administered faster.98 The doses used are the same as those administered for the treatment of severe asthma. It is not clear how far in advance omalizumab should be started before SOTI is initiated, or which diagnostic test should be used to evaluate its effect. In this context, the specific IgE titers are of little use, since the patient is receiving treatment with omalizumab; consequently a significant decrease in prick test size of up to 50%, or its negative conversion, could be used as an indication that omalizumab is achieving the desired effect, and that SOTI therefore can be started. Another aspect that remains to be clarified refers to the period during which omalizumab should be maintained once SOTI has been completed, and whether suspension of the monoclonal antibody should be sudden or gradual. It is not known whether the immunological changes which are beginning to be described in association to SOTI would be the same in the presence of concomitant therapy with an anti-IgE agent such as omalizumab. Lastly, it cannot be discarded that adverse food reactions might occur after suspension of the treatment. The Summary of Product Characteristics of omalizumab does not contemplate food allergy as a therapeutic indication. Consequently, before the monoclonal antibody is used for this purpose, due informed consent should be obtained from the patients or their legal representatives. At present, many clinical trials are evaluating omalizumab in application to food allergy; as a result, in the coming years, and once all the aforementioned doubts have been resolved, it might become a genuine therapeutic option.99,100
Preventive vaccines in children with allergy to egg proteinsThere has always been great controversy regarding the vaccination of children with egg allergy, due to the possibility that such vaccines might contain small amounts of egg as a result of the manufacturing process involved. Three types of vaccines are cultured in chicken egg derivatives: the triple viral vaccine, the influenza vaccine, and the yellow fever vaccine. The only antirabies vaccine marketed in Spain is cultured in human diploid cells, not in chicken embryos.
Triple viral vaccine (MMR)This vaccine is cultured in fibroblasts derived from chicken embryos; it therefore does not contain enough egg protein to trigger an allergic reaction. Consequently, all children with egg allergy, even with clinical manifestations of anaphylaxis, should receive this vaccine in their usual vaccination centre. Those children who have had a reaction with a previous dose of triple viral vaccine should be evaluated by a paediatric allergologist. These reactions occur as a result of allergy to some other components of the vaccine, such as gelatin or neomycin.
The Spanish vaccination calendar recommends administration of the first triple viral vaccine dose at 12 months of age. At this age some infants have still not started to consume egg, but this does not mean that the vaccine should not be administered, since it is not contraindicated in children with egg allergy.101,102
Influenza vaccineThe Spanish Association of Pediatrics recommends administration of the influenza vaccine to children over 6 months of age belonging to certain risk groups, including asthma, as well as to healthy children living with patients at risk.103 Up to one-third of all children with egg allergy have bronchial asthma,104 and since the influenza vaccine is incubated in chicken embryos inoculated with the different influenza viruses, it may contain traces of ovalbumin ranging from picograms to values as high as 42μg/ml.105 The amount of egg protein present in the vaccines is expressed as the ovalbumin concentration per dose. In children presenting egg allergy without severe anaphylaxis, an influenza vaccine containing less than 0.6–1μg/dose of ovalbumin is considered safe106 and can be administered in a single dose without the need for prior skin testing.107,108 In these cases the vaccine can be administered in the usual vaccination centre, which in turn must be prepared to identify and correctly treat anaphylactic reactions.109–111 A more conservative approach is required in those cases in which the vaccine ovalbumin content is high. In this context, administration of the vaccine fractionated into 1/10 and posteriorly 9/10 after 30min has been shown to be safe.112 The vaccine ovalbumin content below which no anaphylactic reaction is to be expected has not been established.113 Influenza immunisation with vaccines containing low amounts of ovalbumin in patients with egg allergy and severe anaphylactic manifestations, using the mentioned vaccination fractionation protocol, has also been shown to be safe, with no serious adverse effects.114 Another series of children with anaphylactic reactions to egg even used vaccines presenting a high egg content, with no serious complications–thus questioning the need to fractionate the dosage and to perform a prior skin test with the vaccine.114
Whenever possible, it would be advisable to administer a vaccine without egg proteins, though no such vaccines are currently marketed in Spain.
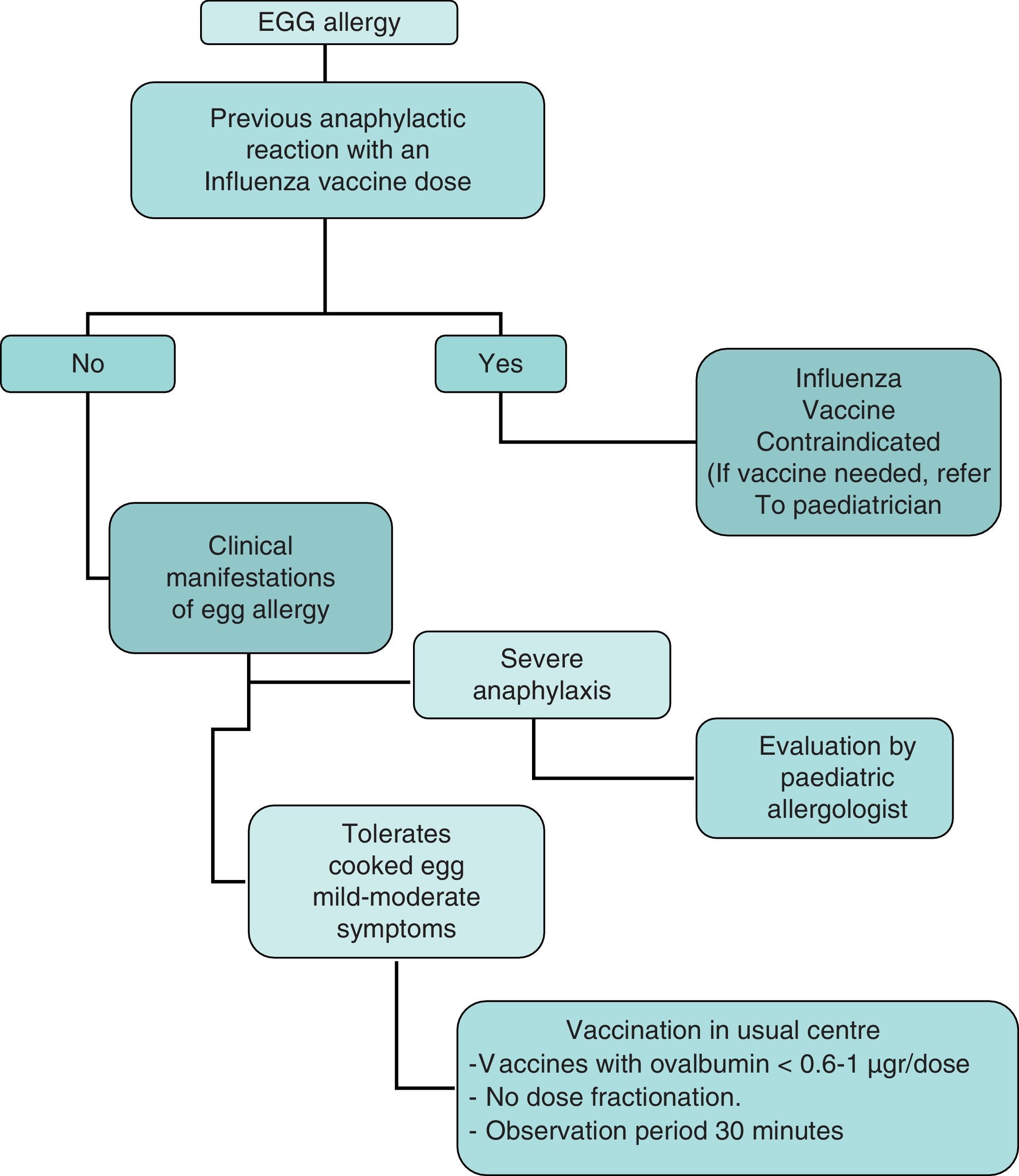
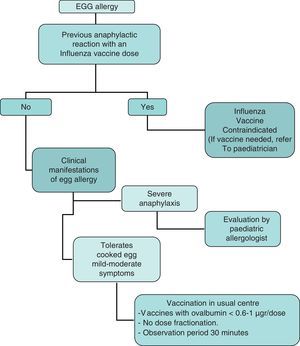
In summary, the recommendations of this Committee for administration of the influenza vaccine to children with egg allergy are the following (Fig. 3):
- 1.
Contraindication of the influenza vaccine in children with severe anaphylactic reactions following the administration of a previous influenza vaccine dose.
- 2.
Contraindication of the influenza vaccine in children with severe anaphylactic reactions after the consumption of egg. If vaccination is considered necessary, it should be administered after due evaluation by the allergologist or paediatric allergologist, in a hospital setting with the means required to adequately deal with anaphylaxis.
- 3.
In the case of non-serious reactions after the consumption of egg, or in children that tolerate cooked egg, influenza vaccination may be carried out with the following specifications:
- 3.1.
The influenza vaccine can be administered in the usual vaccination centre with vaccines presenting an ovalbumin content of under 0.6–1μg/vaccine dose.
- 3.2.
Vaccine dose fraction dose is not needed, and the vaccine can be administered in a single dose.
- 3.3.
A patient observation period of 30min is advised after administration.
- 3.4.
If a second dose is required after 1 month, and there have been no contraindicating reactions, it can be administered as a single dose.
- 3.1.
It would be advisable for the ovalbumin contents of the different influenza vaccines to be stated in the corresponding Summary of Product Characteristics, since the contents may vary from year to year and even from product batch to batch. In any case, most of the vaccines marketed in Spain have very low egg protein contents.
Yellow fever vaccineThis vaccine contains attenuated live viruses, and is therefore not subjected to thermal processing. The viruses are cultured in chicken embryos and can contain significant amounts of egg protein, though the ovalbumin concentration of these vaccines is not known.37 If administration of the yellow fever vaccine proves necessary, the children first should be evaluated by a paediatric specialist with the conduction of an allergy study comprising prick and intradermoreaction tests using a 1/100 dilution of the vaccine. If the test result is positive and vaccination is considered essential, administration should be carried out in a hospital centre with the desensitisation technique.115
The Summaries of Product Characteristics of the aforementioned vaccines state that treatment is contraindicated in patients with allergy to some of the vaccine formulation ingredients, such as egg. On the basis of current knowledge, however, many of these Summaries of Product Characteristics should be modified in order to prevent these children from being needlessly referred to the Paediatric Allergy Department for vaccination, which in many cases can be carried out without problems in the respective primary care centres.
Drugs containing egg proteinsDifferent medicinal products can be formulated with egg proteins, and therefore should be avoided in children with egg allergy.
LysozymeLysozyme is an enzyme with bactericidal activity against anaerobic bacteria, obtained from egg white, although it can also be obtained by biofermentation. In Spain it is found in four drug products, with the description of allergic reactions following their administration. These products are Lysozyme Chiesi®, Lizipaina®, Trofalgon cápsulas® and Rinodexa pediátrico® nose drops.
OvalbuminAlthough no allergic reactions have been reported, Ferroprotina®, Kilor® and Profer® contain ovalbumin bound to ferric products.
As has been commented above, some preventive vaccines contain ovalbumin.
Lecithin/phosphatidesLecithin can come from egg or soya. It is present in propofol and in the lipid emulsion for parenteral nutrition. Propofol is used in the induction of general anaesthesia, and allergic reactions to the drug have been described. It is used in an emulsion containing egg lecithin and phosphatides, soya lecithin, glycerol and sodium hydroxide. There have been isolated reports of anaphylactic reactions in patients with egg allergy. Recently, a paediatric series has confirmed the safety of propofol in cases of patients with non-anaphylactic egg allergy.116,117
The natural course of egg allergyThe prognosis of egg allergy in small children is generally good, though in some cases the condition can persist for years. Moreover, the longer symptomatic sensitisation is maintained, the lesser the probability of resolution – the persistence of clinical reactivity at 9 years of age being an indicator of poor prognosis.118
In three studies carried out in Spain, 50% of the patients with egg allergy reached tolerance at 3–5 years of age, and 64–74% at 9 years of age.5–7
A recent retrospective study carried out in the United States in 881 patients with egg allergy suggests a longer duration of the allergic condition.119 In fact, this study reported a lower proportion of patients who overcame their allergy compared with the above mentioned studies – with a predicted resolution rate of 4% at 4 years, 12% at 6 years, 37% at 10 years, and 68% at 16 years. The risk factors for the persistence of egg allergy were the presence of initial serum specific IgE titers for egg, the presence of other atopic disorders, and the presence of allergy to some other food. The children with a serum specific IgE peak of <2KU/l showed faster development of tolerance.
Elevated specific IgE titers are related to the persistence of egg allergy. In the presence of IgE titers >18–24KU/l for egg white, clinical reactivity is very likely to persist for many years, and in the study published by Savage, patients with serum specific IgE titers >50KU/l were unable to develop tolerance of egg at 18 years of age.37,126
When to assess the evolution of sensitisationIt is advisable to monitor sensitisation every 1–2 years, with a view to assessing the possibility of inducing tolerance through controlled exposure testing.
Levels predictive of toleranceNo sufficiently sensitive or specific clinical or serological parameters have been established yet to allow us to know when tolerance will be reached, although there are some data that can provide a clue. In this context, skin tests can remain positive in 50% of all tolerant individuals, although negative test findings are a good indicator of tolerance.35
Shek et al., in a group of children with egg allergy, found decreases in the levels of specific IgE against egg white of 50% in 12 months to be associated to a 52% probability of tolerance of egg, with the possibility of reintroducing the food.120
Egg tolerance is very likely when the serum specific IgE levels drop to <2KU/l in patients with atopic dermatitis,49 while in patients without atopic dermatitis specific IgE>1.2KU/l is indicative of a high probability of positivity with the provocation test.121
A study of 108 patients with egg allergy aged 35 months on average showed low levels of specific IgE against egg white and ovomucoid to be associated to tolerance of cooked egg. The positive reactivity cut-off point for raw egg, based on a specificity of 95%, was 7.4KU/l for egg white, while the negative reactivity cut-off point, based on a sensitivity of 95%, was 0.6KU/l. In the case of tolerance of cooked egg the levels were higher, with positivity above 10.8KU/l and negativity from 1.2KU/l, for ovomucoid, even if the child proved reactive to raw egg.122
A study carried out in Switzerland involving 35 children with egg allergy evaluated the correlation between specific IgE against egg white and the severity of the reactions in exposure tests of these children. The patients with negative test results presented IgE values of between 0.35 and 6.41KU/l, while those with mild or moderate reactions presented values of 0.35 and 14.0KU/l, and those with severe reactions presented levels between 1.2 and 11KU/l. The authors determined a positivity cut-off point of 17.4KU/l, with a positive predictive value of 95%, for egg white, and of 8.2KU/l, with a positive predictive value of 90%.123
In Spain, a prospective study of 157 children under 16 years of age (mean age 2.6 years) showed skin testing and specific IgE levels (CAP system) to be useful for predicting the persistence of IgE-mediated allergy to egg, particularly in relation to egg white. It is advised not to use oral provocation when the skin test readings are >7mm and/or the specific IgE titers against egg white are >1.3KU/l, since in such situations the probability of persistent allergy is 90%.124
The study conducted in Spain by Montesinos et al.6 quantified specific IgE against egg white with a view to establishing the cut-off point of the persistence of egg allergy and to avoid possibly unnecessary exposure-tolerance testing. These authors took into account the age factor in relation to specific IgE against egg white titers to predict clinical reactivity or tolerance. They concluded that provocation testing is not indicated in the case of cut-off values of >0.35KU/l in children under 2 years of age (positive predictive value (PPV) 92%), 1.52KU/l in children between 2 and 3 years of age (PPV 100%), 1.35KU/l in those between 3 and 4 years of age (PPV 100%), 2.59KU/l in children between 4 and 5 years of age (PPV 100%), and 1.84KU/l in patients over 5 years of age (PPV 100%) – thereby contributing to avoid the need for provocation tests in an important number of children.
We know that children with allergy to cow's milk proteins must be closely monitored, since they are at high risk of suffering IgE-mediated allergy to egg, even before the latter is introduced in the diet. This was evidenced in another study by the same Spanish group, involving 104 infants with allergy to cow's milk proteins, where concomitant egg allergy was documented in 36.5% of the cases (38 infants). The authors determined the predictive value of the skin tests before first exposure to egg, and showed the optimum diagnostic cut-off point to be 6mm for egg white and 5mm for ovomucoid, prior to first exposure to egg proteins, which occurred at 14 months of age.13
Patients with allergy to egg are first able to tolerate cooked egg and raw egg afterwards. When indicated, it is therefore advisable to first perform exposure testing to cooked egg, followed later on by exposure to raw egg.131 It has been suggested that specific IgE titers against ovomucoid are predictors of tolerance of cooked egg, and in this context Ando et al.129 found a concentration of <1KU/l to imply a low risk of reaction to cooked egg white, although the patient may show an intense reaction to raw egg white.
Järvinen et al.125 showed IgE antibodies targeted to sequential epitopes of ovomucoid to be more frequent in patients with persistent egg allergy. The four major epitopes identified in ovomucoid, and which could be used as a screening tool for evaluating the persistence of allergy versus transient allergy, were AA 1–10, 9–20, 47–56 and 113–124. The appearance of IgE against these “informative” epitopes takes place very early in life and could afford important information, with the possibility of applying screening from the time of the initial diagnosis in infants.
The predictive usefulness of serum specific IgE should not be regarded as an absolute criterion but as an orientation, and always taking into account that some patients with IgE levels above the cut-off point are able to tolerate the food. Furthermore, and most importantly, lower values – even titers of <0.35KU/l – particularly in the case of egg allergy, do not necessarily imply that no possibly even serious reactions might occur with exposure testing.
Evolution of allergy: risk of respiratory allergiesThe development of specific IgE against egg during the first year of life is a predictor of the risk of atopic disease. Different studies indicate that immune reactivity to egg may presently be the main and earliest serological marker of the risk of posterior sensitisation to aeroallergens and of the development of respiratory allergic disease.126–130
At this age, the combination of a positive family history (antecedents of atopic disease in at least one first-degree relative) and specific IgE against egg white >2KU/l, is an indicator of future sensitisation to aeroallergens, with high specificity (99%) and positive predictive value (78%).131 If sensitisation proves persistent (more than a year), there is a high risk of developing asthma (67%) and rhinitis (50%) at 5 years of age.138
Furthermore, when egg allergy is associated to atopic dermatitis, the risk of respiratory allergic disease at 4 years of age reaches 80%.8
PreventionPrimary preventionThe exclusion of egg from the maternal diet during breastfeeding (combined with the exclusion of cow's milk and fish), and a delay in its introduction in the diet of the infant up to 2 years of age, appear to reduce the appearance of atopic dermatitis, although without modifying the posterior appearance of allergy to such food and of other allergic disorders.132,133
Until very recently, in the case of infants with a family history of allergy, the expert guides recommended a delay in the introduction of allergenic foods (including the avoidance of egg up to 2 years of age, and of nuts up to 3 years of age), along with a delay in the introduction of complementary feeding from 6 months of age.134 These recommendations could have an opposite effect, however, and at present it is postulated that such measures not only fail to afford protection but may have contributed to the apparent increase in food allergy during the last 20 years.135
A recent survey suggests that the introduction of cooked egg at 4–6 months of age may protect against the development of egg allergy, and that a delay in introduction to 10–12 months may exacerbate the condition.136 This is consistent with the new concept of the possible existence of a “window period” during which exposure to potential allergenic foods facilitates the development of persistent oral tolerance.2
Secondary preventionSensitisation to egg before it is introduced in the diet has been observed in 30–62.5% of all infants with allergy to cow's milk, and in 61% of the infants with atopic dermatitis.11,13,14 Between 44% and 57% of these sensitised infants were seen to develop positive provocation tests with egg at 15 months of age.13,14
In nursing infants with atopic dermatitis or with allergy to cow's milk proteins, skin testing with egg may help identify those children that will suffer an adverse reaction to first introduction of this food in the diet.13,16 In those patients with positive skin testing for egg, a controlled provocation test should be carried out before it is introduced in the diet.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors have no conflict of interest to declare.