Strategies to prevent or reduce the risk of allergic diseases are needed. The time of exclusive breastfeeding and introduction of solid foods is a key factor that may influence the development of allergy. For this reason, the aim of this review was to examine the association between exposure to solid foods in the infant's diet and the development of allergic diseases in children. Classical prophylactic feeding guidelines recommended a delayed introduction of solids for the prevention of atopic diseases. Is it really true that a delayed introduction of solids (after the 4th or 6th month) is protective against the development of eczema, asthma, allergic rhinitis and food or inhalant sensitisation?
In recent years, many authors have found that there is no statistically significant association between delayed introduction of solids and protection for the development of allergic diseases. Furthermore, late introduction of solid foods could be associated with increased risk of allergic sensitisation to foods, inhalant allergens and celiac disease in children. Tolerance may be driven by the contact of the mucosal immune system with the allergen at the right time of life; the protective effects seem to be enhanced by the practice of the breastfeeding at the same time when weaning is started.
Therefore, recent guidelines propose a “window” approach for weaning practice starting at the 17th week and introducing almost all foods within the 27th week of life to reduce the risk of chronic diseases such as allergic ones and the celiac disease. Guidelines emphasize the role of breastfeeding during the weaning practice.
Atopic diseases, such as eczema, asthma, allergic rhinitis and food allergy are common in childhood. Is there any relationship between weaning and allergy? The timing of introduction of solids to infants is an important issue of discussion between paediatricians. When should I start weaning for my child? This is also a frequent question from parents to paediatricians. The age when infants are introduced to solid foods varied greatly during the last half century. The aim of this review is to analyse data from literature evaluating the evidence of the effect of early solid feeding on the risk for allergic diseases in infancy.
The mucosal immune system: tolerance or anergy?Before further discussing the timing of weaning, it is important to consider what the role of the gastrointestinal system is in the induction of tolerance.1 Tolerance could be defined by a lack of reactivity to an antigen/allergen. Tolerance refers to a permanent immunologic state in which infrequent and repeated antigen exposures do not result in an allergic reaction. Tolerance is the usual default response to an allergenic stimulus from the first days of life. However, it can also be induced, for example, by immunotherapy and associated with increased regulatory T cell numbers and increased IL-10 production (explained above). Whether oral desensitisation to foods could be associated with long-term tolerance to a food antigen remains to be elucidated.2
The gut-associated lymphoid tissue is the largest immune organ of the body, the primary route by which we are exposed to antigens. Lamina-propria dendritic cells (DCs) are crucial for the induction of tolerance to intestinal antigens, such as foods. Food proteins and products of commensal bacteria are taken up by DCs and, in the absence of inflammation, driven by prostaglandin E2 (PGE2) (produced constitutively by mesenchymal cells and macrophages), which, through the transformation of growth factor-β (TGF-β) and perhaps interleukin-10 (IL-10), result in the partial maturation of DCs in Peyer's patch or lamina propria. The antigen is presented to naive CD4+ T cells in the mesenteric lymph nodes (MLN), or Peyer's patches. These T cells differentiate into regulatory T cells, which produce IL-10 and interferon-γ (IFN-γ), and/or T helper (Th) 3 cells, which produce TGF-β.3
The result of the interactions between intestinal contents, unique anatomical features (Peyer's patch), and immune and non-immune cells is an environment that favours the induction of IgA.4,5
Dietary protein antigens interact with specific antigen-presenting cells (APCs) that help to activate regulatory T cells, which usually results in the suppression of an immune response. Thus, oral tolerance is the specific suppression of cellular or humoral immune responses to an antigen by means of prior administration of the antigen through the oral route. The response likely evolves as an analogue of self-tolerance to prevent hypersensitivity reactions to food proteins.6
Ingested dietary proteins are broken down and their conformational epitopes are destroyed by gastric acids and luminal digestive enzymes, which often results in the destruction of immunogenic epitopes. Dietary proteins that escape proteolysis in the gut can be taken up by intestinal epithelial cells.1 Several factors, including antigen properties, route of exposure, genetics and age of the host, contribute to the development of oral tolerance.1 The age of exposure is also important in the development of tolerance. In animal models, feeding weight-related doses of ovalbumin to either neonates or adults for the first time resulted in dramatically different outcomes.1
One of the major advances in our understanding of oral tolerance in recent years has been identification of the role of CD103+ (αE integrin) gut DCs and retinoic acid in the induction of oral tolerance.3 Conditioning of gut dendritic cells (DCs) by the gut epithelial cells and the gut flora, which itself has a major influence on gut immunity, induces CD103+ retinoic acid-dependent DC that in turn influences Treg cells production. Th3 type Tregs are transforming growth factor-β dependent and express latency-associated peptide (LAP) on their surface and were discovered in the context of oral tolerance.
There are two primary effector mechanisms for inducing oral tolerance, since active suppression by regulatory T cells may result in clonal anergy or deletion. The primary factor that determines which process will take place is the dose of the antigen. The presence of lower doses of antigen favours tolerance driven by regulatory cells; high doses influence anergy-driven tolerance. Defects in regulatory T-cell activity likely contribute to the development of food allergy.1
The human neonatal gut at birth is immature and breast milk contains several functional nutrients that provide the microenvironment for gut protection and maturation.7 Examination of a cross-section of small intestine in the human foetus shows an immature epithelium, delayed enterocyte proliferation and sparse lymphoid cells. In contrast, examination of the same section of small intestine in the infant that has begun to ingest breast milk reveals an actively proliferating, mature epithelium with all subclasses of enterocytes represented and an abundance of lymphoid tissues.8
Therefore, development of immune tolerance is a critical process in early life. The rising rates of allergic and autoimmune diseases highlight the susceptibility of these tolerance pathways to environmental changes. Delays either in colonisation or in antigen/allergen exposure can lead to failure of oral tolerance.9 Conversely, too early exposure of allergen, when gut colonisation and local immune networks are less established, may increase the risk of allergic or autoimmune diseases.10
In this situation there is also evidence that breastfeeding during the introduction of complementary foods may be relevant for promoting tolerance.11
The classical “old” messages on weaningIn the 1970s a few studies showed an increased risk for eczema and possibly asthma in babies who were introduced to solid foods very early. In a 10-year longitudinal study, Ferguson et al. observed that early exposure (before four months of life) to a varied solid food diet may predispose susceptible children to recurrent or chronic eczema.12
Zeiger et al. reported the results of a randomised, controlled trial that early (before fourth month) combined maternal and infant allergen avoidance of food antigens significantly reduced the risk of eczema in children of atopic parents.13
As a result, in the early 2000s a series of international guidelines recommended late, restricted weaning, especially in high risk infants.14
A joint statement by the European Society for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on hypoallergenic formulas and by the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on nutrition advised postponing the introduction of solid foods to infants – beyond six months of age – to prevent atopic diseases.15 At the same time, a position statement from the American Academy of Pediatrics (APP) recommended withholding cow's milk until the age of one year, eggs until the age of two years, and peanuts, tree nuts and fish until the age of three years, particularly in high risk children.16 In 2001, the World Health Organization limited their recommendations and proposed exclusive breastfeeding for the first six months of life and the introduction of solids only thereafter, even in not-at-risk infants.17 And even a more recent consensus document from the American College of Allergy, Asthma, and Immunology, emphasizing the need for specific practical guidelines for parents and health professionals, suggested that in high-risk infants the introduction of dairy products should be delayed until 12 months of age, eggs until 24 months and peanuts, tree nuts, fish and seafood (fishes and shellfish) until three years of age.18,19
Weaning and allergy: the “new” findingsIn this paper, we use the term ‘complementary feeding’ to embrace all solid and liquid foods other than breast milk or infant formula and follow-on formula, as suggested by ESPGHAN.20
There is mounting concern that the “classical-old” recommended practices of delaying the introduction of complementary foods beyond six months of age may increase, rather than decrease, the risk of immune disorders. Tolerance to food allergens appears to be driven by regular, early exposure to proteins during a “critical early window” of development.21
The window of vulnerability in early infancy is the optimal period when interventions may be targeted and when strategies aimed at reducing the incidence of allergic diseases may be applied.22 Although the timing of this window to prevent allergies is not completely clear, current evidence suggests that this is most likely to be allocated between the 4th (around 17 weeks) and the 7th month of life, and that particularly delayed exposure to solid foods beyond this period may increase the risk of food allergy, celiac disease and islet cell autoimmunity.21
In 2008, AAP explained that food introduction depends on child readiness at his own rate of development. The child is ready when he holds his head up, he opens his mouth when food comes his way, he moves food from a spoon into his throat, when infants double their birth weight (typically at about four months) they may be ready for solid food introduction.23 AAP update in 2012 underlines that there is no evidence that introducing “allergenic foods” (like eggs and fish) after four to six months of age determines whether your baby will be allergic to them.23
As previously demonstrated in ‘classical’ reports, very early introduction of solid foods may result in allergic sensitisation against (food) allergens, because the infant's gut mucosal barrier is immature and early exposure may trigger an allergic response of the immune system.1 However, since no increased risk for the development of food allergies was found in a study of children with an immature gastrointestinal tract or immune response, there is only scarce scientific evidence to support this hypothesis.24 A number of subsequent prospective studies have failed to demonstrate an association between early introduction of complementary foods and either eczema or food allergy, while a systematic review concluded that there was “no strong evidence to support the association between early solid feeding and the development of persistent asthma, persistent food allergy, and allergic rhinitis”.25
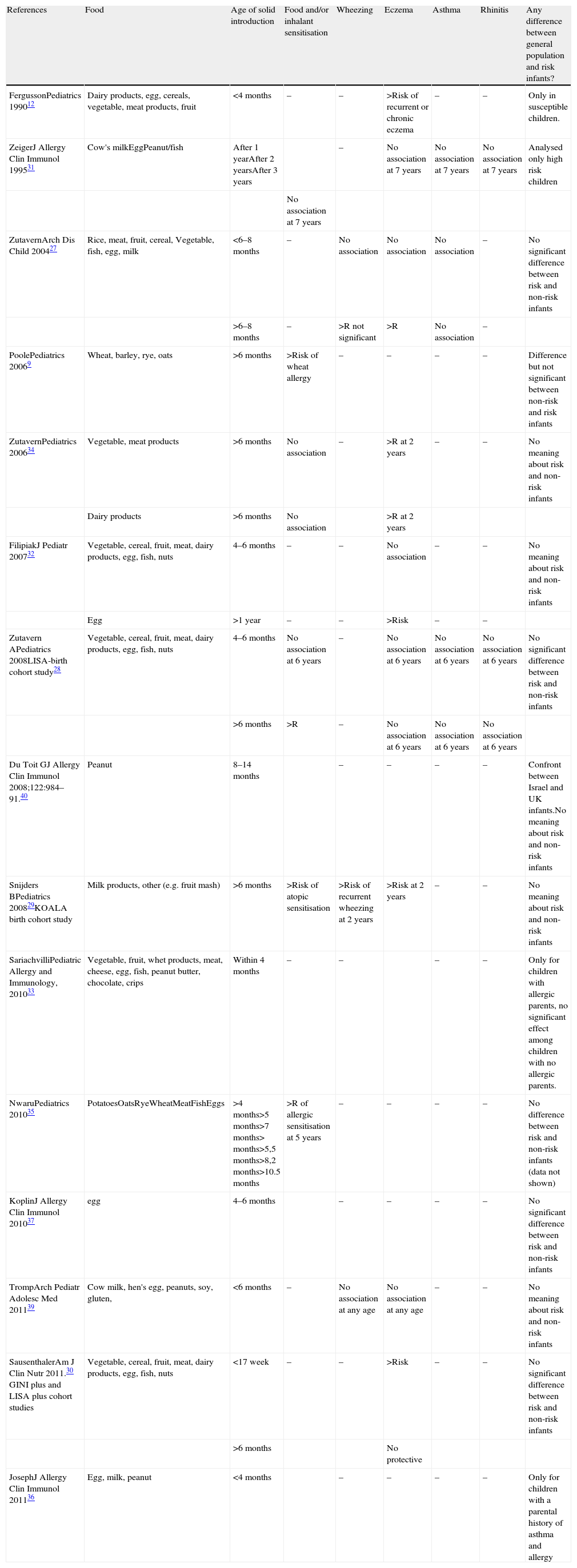
Some prospective studies supported neither prolonged breastfeeding nor delayed introduction of solid foods as protective measurements against the development of allergic diseases in children.26–32 Conversely, an increased risk of atopic dermatitis, eczema and allergic sensitisation (with or without symptoms) has been associated with delayed introduction of eggs, milk, cereals and other solids27,29,33 (see Table 1).
Correlation between food, time of food introduction and allergic diseases.
| References | Food | Age of solid introduction | Food and/or inhalant sensitisation | Wheezing | Eczema | Asthma | Rhinitis | Any difference between general population and risk infants? |
| FergussonPediatrics 199012 | Dairy products, egg, cereals, vegetable, meat products, fruit | <4 months | – | – | >Risk of recurrent or chronic eczema | – | – | Only in susceptible children. |
| ZeigerJ Allergy Clin Immunol 199531 | Cow's milkEggPeanut/fish | After 1 yearAfter 2 yearsAfter 3 years | – | No association at 7 years | No association at 7 years | No association at 7 years | Analysed only high risk children | |
| No association at 7 years | ||||||||
| ZutavernArch Dis Child 200427 | Rice, meat, fruit, cereal, Vegetable, fish, egg, milk | <6–8 months | – | No association | No association | No association | – | No significant difference between risk and non-risk infants |
| >6–8 months | – | >R not significant | >R | No association | – | |||
| PoolePediatrics 20069 | Wheat, barley, rye, oats | >6 months | >Risk of wheat allergy | – | – | – | – | Difference but not significant between non-risk and risk infants |
| ZutavernPediatrics 200634 | Vegetable, meat products | >6 months | No association | – | >R at 2 years | – | – | No meaning about risk and non-risk infants |
| Dairy products | >6 months | No association | >R at 2 years | |||||
| FilipiakJ Pediatr 200732 | Vegetable, cereal, fruit, meat, dairy products, egg, fish, nuts | 4–6 months | – | – | No association | – | – | No meaning about risk and non-risk infants |
| Egg | >1 year | – | – | >Risk | – | – | ||
| Zutavern APediatrics 2008LISA-birth cohort study28 | Vegetable, cereal, fruit, meat, dairy products, egg, fish, nuts | 4–6 months | No association at 6 years | – | No association at 6 years | No association at 6 years | No association at 6 years | No significant difference between risk and non-risk infants |
| >6 months | >R | – | No association at 6 years | No association at 6 years | No association at 6 years | |||
| Du Toit GJ Allergy Clin Immunol 2008;122:984–91.40 | Peanut | 8–14 months | – | – | – | – | Confront between Israel and UK infants.No meaning about risk and non-risk infants | |
| Snijders BPediatrics 200829KOALA birth cohort study | Milk products, other (e.g. fruit mash) | >6 months | >Risk of atopic sensitisation | >Risk of recurrent wheezing at 2 years | >Risk at 2 years | – | – | No meaning about risk and non-risk infants |
| SariachvilliPediatric Allergy and Immunology, 201033 | Vegetable, fruit, whet products, meat, cheese, egg, fish, peanut butter, chocolate, crips | Within 4 months | – | – | – | – | Only for children with allergic parents, no significant effect among children with no allergic parents. | |
| NwaruPediatrics 201035 | PotatoesOatsRyeWheatMeatFishEggs | >4 months>5 months>7 months> months>5,5 months>8,2 months>10.5 months | >R of allergic sensitisation at 5 years | – | – | – | – | No difference between risk and non-risk infants (data not shown) |
| KoplinJ Allergy Clin Immunol 201037 | egg | 4–6 months | – | – | – | – | No significant difference between risk and non-risk infants | |
| TrompArch Pediatr Adolesc Med 201139 | Cow milk, hen's egg, peanuts, soy, gluten, | <6 months | – | No association at any age | No association at any age | – | – | No meaning about risk and non-risk infants |
| SausenthalerAm J Clin Nutr 2011.30 GINI plus and LISA plus cohort studies | Vegetable, cereal, fruit, meat, dairy products, egg, fish, nuts | <17 week | – | – | >Risk | – | – | No significant difference between risk and non-risk infants |
| >6 months | No protective | |||||||
| JosephJ Allergy Clin Immunol 201136 | Egg, milk, peanut | <4 months | – | – | – | – | Only for children with a parental history of asthma and allergy |
In 2004, for the first time, Zutavern et al. showed an increased risk of eczema related to the late introduction of eggs and milk in a prospective birth cohort study.27 There was a statistically significant increased risk of eczema in relation to the late introduction of these foods. The late introduction of eggs was associated with a non-significant increased risk of preschool wheezing.27
In 2006, the same authors in another birth cohort study showed that late introduction of vegetables and meat products increased the risk of physician-diagnosed atopic dermatitis in two-year-old children; whereas late introduction of dairy products increased the risk of symptomatic atopic dermatitis. No association with atopic sensitisation was observed.34
Poole et al. showed that children who were first exposed to cereals after six months of age had an increased risk of wheat allergy compared to children who were first exposed to cereals before six months of age.9 In 2008, Zutavern et al., studying another cohort of 2073 children at six years of age, demonstrated that delayed introduction of solids (beyond six months of age) was not associated with a decreased odds ratio for asthma, allergic rhinitis or sensitisation against food or inhalant allergens at five years of age. On the contrary, food sensitisation was more frequent in children who were introduced to solids later as infants.29
Nwaru et al. showed that allergic sensitisation to any food allergens was associated with the late introduction of potatoes, oats, rye, meat, fish and eggs (beyond four months of age). Similarly, sensitisation to any inhalant allergens was associated with the late introduction of potatoes, oats, rye, meat and fish.35
Certainly, some foods are more allergenic than others, for example egg and peanut.21,36 However, infant introduced to egg at four to seven months had a lower risk of egg allergy than those introduced to egg after that time, particularly those introduced to egg at 10–12 months of age and after 12 months of age, even after adjusting for family history of allergy and infant allergy symptoms. The lower risk of egg allergy was found among infants whose first exposure to egg occurred at four to six months of age in the form of cooked egg compared with those introduced later.37
The lowest risk of egg allergy occurred among infants exposed to cooked egg between four and seven months of age is consistent with the new concept of a window of opportunity during which exposure to potentially allergenic foods promotes the development of persistent oral tolerance.21
Alternatively, continued exposure to low doses of egg from four to six months of age might have induced desensitisation in infants who previously had undiagnosed egg allergy by the time egg challenges were conducted after 12 months of age, as seen in early reports of egg oral immunotherapy for the treatment of egg allergy.38
According to Tromp et al., the introduction of tree nuts before the age of six months was significantly associated with wheezing at two years of age, not at three years of age. In addition, no significant association was found between early introduction to tree nuts and eczema up to age four years. This association was explained by sex, maternal smoking, gestational age, race/ethnicity, birth weight, parity, breastfeeding, use of any antibiotics, day care attendance, gastroenteritis, number of respiratory tract infections, overweight and parental history of atopy. The introduction of cow's milk, hen's egg, peanut soy and gluten to an infant's diet before the age of six months was not associated with wheezing or eczema at any age. Some limitations of the study have to be considered in the interpretation of the results. First, eczema and wheezing were diagnosed on the basis of parent-reported questionnaires. Second, the effect of allergenic food introduction was not examined before the age of four months in relation to eczema and wheezing.39
Joseph et al., in a 594 children's cohort, demonstrated that complementary food introduced before four months was associated with a reduced risk of peanut (and perhaps egg) sensitisation by age two to three years, but only for children with a parental history of asthma or allergy.36
Finally, Du Toit et al. showed that despite precise guidelines recommending avoidance of peanuts during infancy, which are strictly applied in the United Kingdom, Australia and North America, peanut allergy continues to increase in these countries; whereas this sensitisation is decreasing among children from Israel.40
Since the median consumption of peanut products in Israel for infants aged 8–14 months is 7,1g/month, and 0g/month in the UK (p<0,01), it is fascinating to hypothesize that early introduction of peanuts during infancy, rather than strict avoidance, would prevent the development of peanut allergy.40
Venter et al. show that peanut sensitisation and reported allergy in children born in 1994–1996 increased from 1989 but seems to have stabilised or slightly decreased since the late 1990s, although not significantly.41
Amin et al. in a cohort of patients diagnosed with “food allergy” from 2003 to 2008 demonstrated that the percentage of peanut allergic children in 2008 was slightly larger than in 2003 but this difference was not statistically significant. According to these authors it is possible that the institution of guidelines based on best practices at that time (delayed introduction of peanuts after three years of age), may have contributed to an increase in prevalence and severity of food allergy in their cohort. Further study would be required with follow-up investigation to determine the importance of this effect.42
At present, there are two ongoing studies on the role of early antigen administration in the development of allergic diseases – the LEAP and the EAT studies. The results of these studies will help to clarify the role of the timing of allergen exposure in the manifestations of the disease.43,44
Prevention of celiac disease: the role of complementary feedingRecent epidemiological studies have suggested that early or too late infant feeding practices may be important risk factors also for the subsequent development of other chronic diseases and particularly of celiac disease (CD).45,46
Breastfeeding may offer protection during the introduction of dietary gluten, and increasing the duration of breastfeeding has also been associated with reduced risk of developing CD.45
Experience from Sweden showed a sharp increase in cases of celiac disease after advice was taken to delay the introduction of gluten from before four months to beyond six months of life.47
Ivarsson et al. studied the epidemiology of this epidemic and found that the risk of developing CD was reduced in children younger than two years if they were still being breastfed when dietary gluten was first introduced.11
The actual mechanisms through which breast milk protects against the development of CD are unclear. It could be argued that continuing breastfeeding at the time of weaning limits the amount of gluten that the child receives, thereby decreasing the chances of the child developing gluten hypersensitivity.45 Another mechanism through which breast milk could offer protection is by preventing gastrointestinal infections in the infant. Infections of the gastrointestinal tract in early life can lead to increased permeability of the intestinal mucosa, allowing the passage of gluten into the lamina propria.48
Juto et al. suggested two other possible mechanisms. First, human milk IgA antibodies may diminish an immune response to ingested gluten by mechanisms such as agglutination of the antigen to immune complexes on the mucosal surface, so that uptake is prevented. Second, the immune modulating property of human milk may be exerted though its T cell specific suppressive effects as shown by experiments on peripheral lymphocytes stimulated with phytohaemagglutin, OKT3 and alloantigens.49
According to Norris et al., children not exposed to wheat, barley or rye until their seventh month or later were at an increased risk of CD compared with those who were exposed during the four to six month period. The reason why late gluten exposure is also associated with CD is less clear. When wheat is introduced to an older child, it tends to be introduced in greater amounts, thus increasing the amount of gliadin available to cross the gut. The authors concluded that both the too early (before four months) and too late (beyond seven months) introduction of cereals containing gluten were associated with an increased risk of CD.50
The results of these studies are subject to limitations, particularly because they are case control studies, which are influenced by recall bias (e.g. misclassification of breastfeeding, the age of introduction of gluten) and other confounding factors such as socioeconomic status and HLA genotypes.47 On the basis of current data, the ESPGHAN Committee considers it wise to avoid introducing gluten either too early (before four months) or too late (beyond seven months), and recommends introducing small amounts of gluten gradually, especially when the infant is still being breastfed.21
ConclusionsDespite the paucity of clear evidence, conservative delay/avoidance recommendations remain in place in many countries. ESPGHAN recently issued a position paper on complementary feeding which states that avoidance or delayed introduction of allergenic foods for the purpose of avoiding allergies is not recommended. They do, however, recommend the avoidance of very early (before four months of age) or too late (after seven months of age) introduction of gluten to reduce the risk of allergy or CD.20 Likewise, other national guidelines propose the same approach that now must be extended to the global practice of paediatricians.22,23 There is no convincing scientific evidence that avoidance or delayed introduction of potentially allergenic foods, such as fish and eggs, reduces allergies either in infants considered at increased risk for the development of allergy, or even in those not considered to be at increased risk (see Box 1). This must also be applied to infants with siblings who already have allergies.
Main messages.
Exclusive breastfeeding for at least 4 months and continuing breastfeeding during weaning onto solid foods decreases risk of atopic diseases
No starting weaning before 17 weeks (increased risk of atopic diseases)
No starting weaning after 26 weeks (increased risk of atopic diseases); no difference in high-risk and in non-risk infants
No evidence that weaning is different between risk and non-risk infants, but delayed introduction of solids, especially in high risk infants could increase the risk of atopic diseases
Further studies are needed to prove the development of the tolerance by food exposure (e.g. LEAP and EAT study)
There are some suggestions stating that delaying the introduction of certain foods may actually increase (rather than decrease) the prevalence of allergic diseases. Therefore, when a child is ready, from the fourth month of life, a new solid food should be introduced every couple of days, while encouraging the mother to possibly continue breastfeeding during this period.20,22
Ethical disclosuresProtection of human subjects and animals in researchThis is a review and there is no concern regarding human subjects and animals in research.
Patients’ data protectionThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interestThe authors have no conflict of interest to declare.