Food protein-induced enterocolitis syndrome (FPIES) is a food hypersensitivity disorder, characterised by severe vomiting and/or diarrhoea.1 Elimination of the offending food from diet is the only therapy. On the contrary, for patients affected by IgE–mediated cow's milk and egg allergy therapeutical options in the last years seem to be different, such as Specific Oral Tolerance Induction (SOTI).2 Additionally, most children affected by IgE-mediated cow's milk and egg allergy can tolerate offending food if well-cooked and mixed with wheat.3–4 SOTI appears perhaps of little advantage for children affected by FPIES, because of its very rare persistence after 3–4 years of age. The liberalisation of diet for the offending–but well-cooked–food should not be possible, too: FPIES, considered a non-IgE-mediated allergy, should not benefit from cooking, which can decrease protein allergenicity in several ways, including the destruction of predominantly conformational epitopes, with limited effect on sequential epitopes.5 Infact, Caubet and Nowak-Wegrzyn6 describe a case of egg FPIES with adverse reaction also after ingestion of a baked cake prepared with egg.
Anyway, the observation of some of our cases and considerations about FPIES immunopathogenesis could induce to better explore the reliability of this hypothesis.
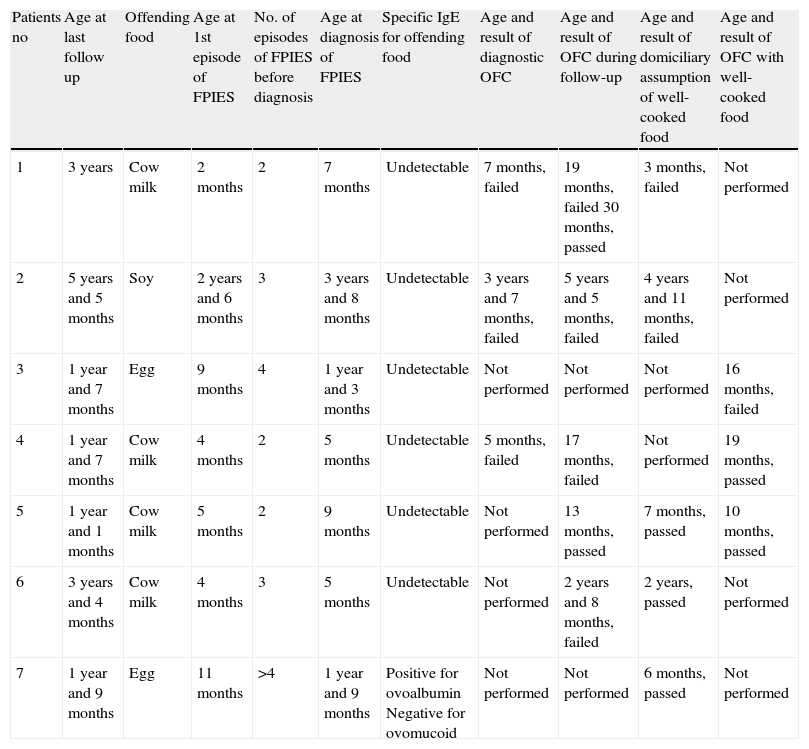
Case seriesTable 1 shows major information on seven children affected by FPIES, who ate offending food well-cooked (baked) spontaneously at home or by oral food challenge (OFC). OFC was conducted with 100g of “ciambellone” (a typical Italian cake) containing 20ml (0.6g of cow's milk protein) of cow's milk, half hen's egg and wheat flour, baked at 180°C for 30min. Diagnosis of FPIES was made following Powell's criteria7; all the children had no other allergic diseases except the FPIES. In two cases diagnosis of “atypical” FPIES was done, because of the advanced age of symptoms occurrence (patient no. 2) or because of the presence of specific IgE against offending food (patient no. 7). Focusing on this last case, skin prick test (ALK-Abellò, Milan) resulted positive for ovalbumin and negative for ovomucoid. 4/7 (57%) patients had tolerance for well-cooked food when allergy to uncooked or poorly-cooked food was still very likely. In particular, patient no. 4 presented a failed OFC with the raw food at 17 months and then he tolerated the well-cooked food at 19 months; patient no. 5 presented the last episode of FPIES at six months and he tolerated the well-cooked food at seven months; patients no. 6 and no. 7 had tolerated the well-cooked food some months before presenting the last episode of FPIES with the offending food uncooked or poorly uncooked. In several cases (5/7) parents spontaneously tried to give offending food well-cooked (for example biscuit or other sweets, always baked) at home. When domiciliary ingestion had been routinely repeated, we did not perform OFC with well-cooked food; OFC was performed for patient no. 5 because offending food well-cooked had been given at home just once. For children who appeared tolerant, free assumption of the offending food, if well-cooked, was allowed. Follow up after liberalisation of the diet for well-cooked foods lasted at least six months, without adverse reactions. “Although FPIES is well established as a distinct clinical entity, its pathophysiology has not yet been clearly defined and requires further characterisation. Several immunologic alterations have been reported in FPIES, suggesting the involvement of antigen-specific T cells and their production of proinflammatory cytokines that regulate the permeability of the intestinal barrier. Humoral immune responses may also be involved in the pathomechanism of FPIES”.1 If pathogenesis of FPIES is erected on an exclusive cell-mediated mechanism, tolerance versus the offending food well-cooked could not be possible in an active FPIES. In fact, denaturation of conformational epitope caused by high and prolonged temperature, should not obstruct antigen-receptor link, as happens in the majority of the cases of IgE-mediated allergies. In fact, receptor of T lymphocytes (TCR) recognise antigenic peptides derived from proteins degraded and exposed on cellular surface of dendritic cells through link with MCH1 receptors present on own surface and not specific for that allergen, but independent from its structural conformation.8 Anyway, some elements could indicate a possible role for specific IgE in pathogenesis of FPIES: (a) time of symptoms occurrence, usually 2h from the ingestion of the offending food is neither typical for cell-mediated reactions, which usually happen later, nor of classic IgE-mediated reactions; it appears to be halfway between IgE- and non IgE-mediated reactions and, moreover, some children present symptoms before 2h; (b) in selected cases of FPIES (3–5%) allergy tests for IgE resulted positive; (c) some cases of FPIES evolve to IgE-mediated gastrointestinal anaphylaxis9; (d) although rare, in the same child FPIES with undetectable IgE for the offending food can coexist with food IgE-mediated allergy for other foods10; (e) specific IgE present only at duodenal site has been found in adult patients with symptoms of a pathology similar to FPIES, apparently not IgE-mediated.11
Major features of patients shown according to result of assumption of the well-cooked food.
| Patients no | Age at last follow up | Offending food | Age at 1st episode of FPIES | No. of episodes of FPIES before diagnosis | Age at diagnosis of FPIES | Specific IgE for offending food | Age and result of diagnostic OFC | Age and result of OFC during follow-up | Age and result of domiciliary assumption of well-cooked food | Age and result of OFC with well-cooked food |
| 1 | 3 years | Cow milk | 2 months | 2 | 7 months | Undetectable | 7 months, failed | 19 months, failed 30 months, passed | 3 months, failed | Not performed |
| 2 | 5 years and 5 months | Soy | 2 years and 6 months | 3 | 3 years and 8 months | Undetectable | 3 years and 7 months, failed | 5 years and 5 months, failed | 4 years and 11 months, failed | Not performed |
| 3 | 1 year and 7 months | Egg | 9 months | 4 | 1 year and 3 months | Undetectable | Not performed | Not performed | Not performed | 16 months, failed |
| 4 | 1 year and 7 months | Cow milk | 4 months | 2 | 5 months | Undetectable | 5 months, failed | 17 months, failed | Not performed | 19 months, passed |
| 5 | 1 year and 1 months | Cow milk | 5 months | 2 | 9 months | Undetectable | Not performed | 13 months, passed | 7 months, passed | 10 months, passed |
| 6 | 3 years and 4 months | Cow milk | 4 months | 3 | 5 months | Undetectable | Not performed | 2 years and 8 months, failed | 2 years, passed | Not performed |
| 7 | 1 year and 9 months | Egg | 11 months | >4 | 1 year and 9 months | Positive for ovoalbumin Negative for ovomucoid | Not performed | Not performed | 6 months, passed | Not performed |
The hypothesis that FPIES can also be IgE-mediated has been formulated by experts on this arguement: “Although a role for IgE in the pathophysiology of the disorder has not been established, it has not been completely excluded”.1 All these elements, in addition to our observations that 4/7 of our children with FPIES tolerated the offending food well-cooked (baked), makes the question eligible for further analysis. Finding specific IgE for ovalbumin, which is a thermolabile egg protein, in child no. 7 makes this hypothesis reliable.
Our children have eaten just a little dose of the offending food. In previous studies3,4 for the elaboration of baked food, freeze–dried milk and/or egg was used, which allowed a more consistent ingestion of cooked protein. Therefore, it could be hypothesised that tolerance for the offending food well-cooked reached in our patients is due to the little amount of food protein eaten, rather than denaturation of the offending epitope cooking-induced. Anyway, if in common life preparation of baked food with freeze–dried milk and/or egg is improbable, improvement in the quality of life for children with FPIES is a eligible goal.
Additionally, similarly to IgE-mediated allergy,12 getting tolerance for well-cooked food could represent a marker to discriminate forms of FPIES which would resolve earlier (see patient no. 5, Table 1) from those which are longer lasting and more difficult to treat.
Finally, we hypothesise that phenotypes of FPIES mediated by specific IgE against the offending food could exist. Some of these children could tolerate the offending food if well cooked because of degradation of the conformational offending epitope. We hope that a better understanding of the physiopathologic basis of FPIES, presentation of other cases and/or results of future prospective studies can support or deny our working hypothesis.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that no patient data appears in this article.
Right to privacy and informed consentRight to privacy and informed consent. The authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors have no conflict of interest.