The reproducibility of the adverse reaction increases the suggestiveness of a history of food allergy. However, the positive predictive value (PPV) of multiple adverse reaction episodes for the diagnosis of IgE-mediated food allergy is not known. This evaluation was the objective of our study.
Patients and methodsWe retrospectively studied 180 children with a history of non-anaphylactic adverse reactions after the ingestion of a food. All children had the prick test positive for the offending food and performed the oral food challenge (OFC) within 12 months after the last adverse reaction episode (ARE). We have evaluated whether increasing the number of ARE increased the probability that the OFC would be positive (failed).
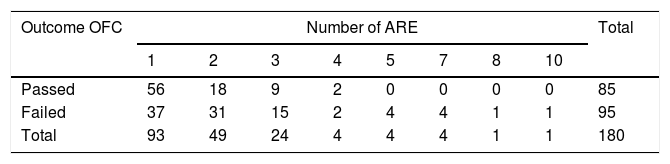
Results93 patients (52%) presented one ARE, 49 (27%) presented two ARE, 24 (13%) presented three ARE, 14 (8%) patients presented≥four ARE. The OFC was positive in 94/180 (52%). The outcome of the OFC was found to be positively correlated with the number of ARE (OR=1.56; 95% CI=1.16–2.09; p=0.003). A PPV=100% was observed with a number of ARE≥five.
ConclusionsThe number of ARE is an important predictor of the diagnosis of food allergy, although less than we would have imagined. The number of ARE could be used to increase the predictability of the diagnostic tests currently in use, to define clinical prediction rules alternative to OFC and easy to use in clinical practice.
The diagnosis of IgE-mediated Food Allergy (IgE-FA) is based on: (a) a suggestive history; (b) a positive allergy test; (c) a positive oral food challenge (OFC) (if appropriate).1,2 The OFC is the gold standard test, it must be performed in hospital and involves a waste of human and economic resources and, above all, risk for the patient. OFC can be avoided when the diagnosis of IgE-FA is very likely: a recent history of anaphylaxis and/or values of the allergy test above certain thresholds are reasonable reasons for issuing the diagnosis of IgE-FA without performing the OFC.3 The necessity of OFC, except in the case of the above two exceptions, is justified by the low positive predictive value (PPV) of the history,4 even if associated with a positive allergy test. To improve its standardization, questionnaires were developed for the collection of the history in case of suspected adverse reaction to foods of allergic nature, such as that proposed in the guidelines for the FA of the European Academy of Allergy and Clinical Immunology (EAACI).2 Among the various factors that can be explored through this questionnaire, there is the reproducibility of the adverse reaction: the fact that the adverse reaction has been repeated several times reinforces the suspect.
However, to our knowledge the PPV of multiple adverse reactions to food has not been studied. The primary objective of this study was to establish whether, in cases of suspected IgE-FA, the increase in the number of adverse reaction episodes (ARE) following the ingestion of a food increases the probability that the OFC will fail.
Patients and methodsWe carried out a retrospective study at the pediatric allergy unit of the Policlinico Gemelli Universitary Foundation IRCCS of Rome, Civil Hospital of Senigallia, Belcolle Hospital of Viterbo, Fatebenefratelli Hospital of Benevento, Burlo Garofolo University Hospital of Trieste, San Camillo Hospital of Rome, Loreto Crispi Hospital of Naples.
We considered all those children who, from 1/1/07 to 12/31/17, carried out an OFC at these hospitals because of a suspected IgE-FA against any food, as being eligible. The suspect of IgE-FA was induced from the positive history for at least one ARE and the positive result of the skin prick test (SPT) with commercial extract or prick by prick (PbP) with the involved food (IF). The medical records of eligible children were searched in the databases of the pediatric allergy units and the following information was taken from their examination: (a) age of the child at the time of the first ARE; (b) sex; (c) any atopic comorbidity (atopic dermatitis, asthma, rhinitis, other food allergies); (d) type of IF; (e) number of previous innocuous ingestion of the food in the involved form (processed or not) in the ARE and time frame during which they occurred; (f) number of ARE; (g) symptoms and signs of each ARE, classified by type and severity (mild, moderate, severe, according to ref 3); (h) amount of IF ingested for each ARE; (i) time of onset of symptoms and signs of each ARE; (j) time of resolution of the symptoms and signs of each ARE; (k) mean diameter of the wheal elicited by the SPT and/or PbP with the IF; (l) date of execution of the OFC and its outcome (failed or passed); and (m) time between last ARE and OFC.
Children with a history compatible with the diagnosis of anaphylaxis, according to Sampson et al.,5 which occurred within 12 months prior to our evaluation, were excluded. In this type of patients, in fact, diagnostic OFC is not usually performed.3 Furthermore, the study excluded children: (a) whose medical records did not contain the minimum information necessary to satisfy the objective of the study, such as the number of ARE and the outcome of the diagnostic OFC; (b) who had performed the diagnostic OFC with a distance of more than 12 months from the last ARE; (c) whose parents denied consent to performing the diagnostic OFC.
All patients who were eligible performed skin allergy test with the IF, always in the form of raw and/or cooked natural food (PbP) and sometimes in the form of a commercial extract (SPT). The SPTs were performed according to an international methodology.6
In all enrolled patients an open OFC was performed and evaluated according to an international methodology.3,7,8
The study was approved by each hospital's ethics committee. Written informed parental consent was obtained before enrollment.
Statistical analysisFor continuous variables, mean±standard deviation (SD), median and range (minimum and maximum) were calculated. For categorical variables the proportions (prevalences) were calculated, both on the total patients and on the subgroups of interest. We used the χ2 test to evaluate the differences between frequencies. Logistic regression analysis was performed to evaluate the possible association of positive OFC with other variables. For each variable, the odds ratio (OR) and 95% confidence interval (95% CI) have been calculated. The 2×2 tables were used to calculate the values of Sensitivity, Specificity, PPV and Negative Predictive Value (NPV), Positive Likelihood Ratio (LR+) and Negative Likelihood Ratio (LR−). The diagnostic accuracy of the numerical variables at various cut-off points was calculated with the ROC (Receiver Operator Characteristics) curves. Cut-off values with PPV≥95% or 100% specificity were considered diagnostic of FA. The p value <0.05 was considered statistically significant. For statistical analysis STATA 13 software was used.
ResultsThe study included 180 patients, 121 males (67%) and 59 females (33%), with suspected IgE-FA. They accounted for about 10% of patients who received a diagnosis of IgE-FA in the period considered and by the participating centers. The reasons for exclusion of about 90% of eligible patients were: (a) more than 12 months of latency between the last ARE and diagnostic OFC (most frequent reason); (b) insufficient documentation in the medical record; (c) diagnostic OFC not performed because of exceeding cut-off of the allergy test.
The mean age±SD at the time of the 1st ARE was 1.8±2.7 years (range of 0.04–12.8 years). Patients with one comorbidity were 107 (59%), 44 (24%) with two comorbidities, 11 (6%) with three comorbidities, two (1%) with four comorbidities. The most frequently found comorbidity was atopic dermatitis (87/180, 48%).
Features of the ARE93 patients (52%) presented one ARE, 49 (27%) presented two ARE, 24 (13%) presented three ARE, 14 (8%) patients presented ≥four ARE. The average number of ARE per patient was 1.92.
Cow's milk was the most frequently IF (53% of ARE), followed by chicken egg (20% of ARE), nuts (6%), wheat (3.5%), peanut (2%), fish (2%). Other foods have been implicated with lower frequency rates. Among the signs and symptoms, the muco-cutaneous manifestations (pruritus, exanthema, urticaria, angioedema) were present in 65% of the ARE, followed by gastrointestinal disorders (20%), and respiratory disorders (15%). Eighty-one patients presented mono-organ ARE and 99 patients presented multi-organ ones: patients with >one ARE maintained the initial mono- or multi-organicity characteristic.
Correlation of the outcome of the OFC with the number of AREsThe OFC was, by definition, carried out on all patients and was positive in 94/180 (52%), of which 45/94 (48%) performed with an interval under six months from the last ARE and 49/94 (52%) performed with an interval between six and 12 months from the last ARE (p=0.98).
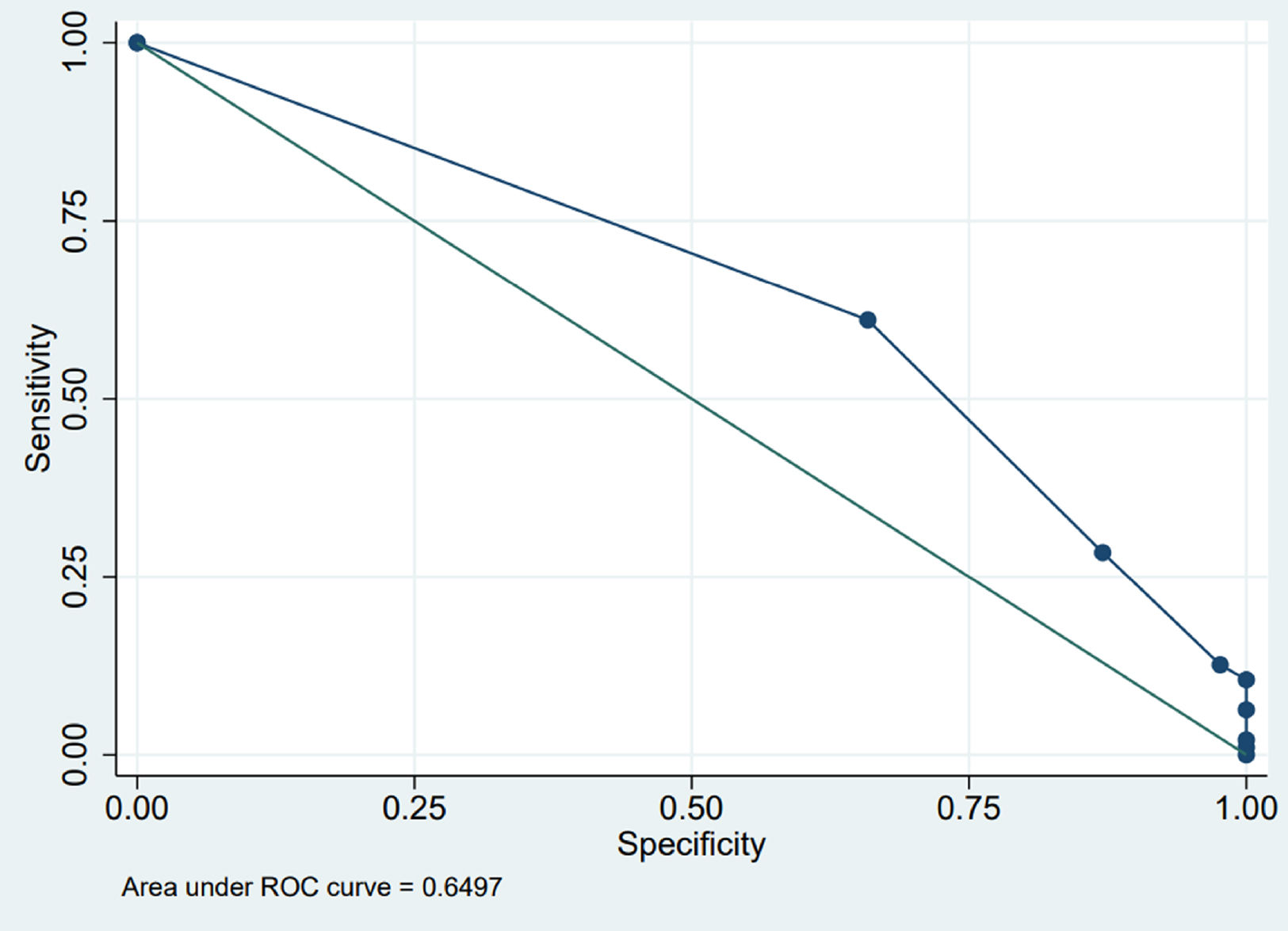
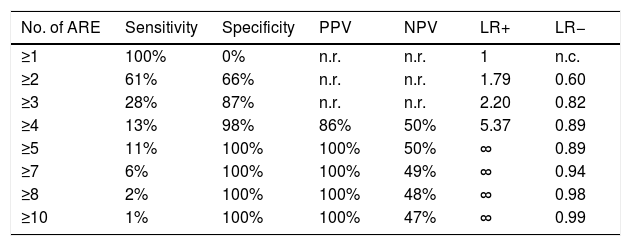
The outcome of the OFC was found to be positively correlated with the number of ARE (OR=1.56; 95% CI=1.16–2.09; p=0.003) (Table 1). The diagnostic accuracy of the test (number of ARE) is shown in Table 2 and Fig. 1. A clinically relevant PPV was observed with a number of ARE≥five (PPV=100%).
Diagnostic accuracy of the number of ARE.
| No. of ARE | Sensitivity | Specificity | PPV | NPV | LR+ | LR− |
|---|---|---|---|---|---|---|
| ≥1 | 100% | 0% | n.r. | n.r. | 1 | n.c. |
| ≥2 | 61% | 66% | n.r. | n.r. | 1.79 | 0.60 |
| ≥3 | 28% | 87% | n.r. | n.r. | 2.20 | 0.82 |
| ≥4 | 13% | 98% | 86% | 50% | 5.37 | 0.89 |
| ≥5 | 11% | 100% | 100% | 50% | ∞ | 0.89 |
| ≥7 | 6% | 100% | 100% | 49% | ∞ | 0.94 |
| ≥8 | 2% | 100% | 100% | 48% | ∞ | 0.98 |
| ≥10 | 1% | 100% | 100% | 47% | ∞ | 0.99 |
ARE=adverse reaction episode; PPV=positive predictive value; NPV=negative predictive value; LR+=positive likelihood ratio; LR−=negative likelihood ratio; n.r.=not reported because it was too low; n.c.=not calculable; ∞=infinite. There were no patients with n. ARE=6 or =9.
ROC curve. The points are the different values of number of ARE. There are eight values, from left to right: 1–2–3–4–5–7–8–10. The first in the upper left is ARE=1, corresponding to sensitivity=1.00 (ordinate), i.e. 100%, and specificity=0.00 (abscissa), i.e. 0%. The last two in the lower right, one above the other, are 8 and 10, corresponding to sensitivity=0.02, i.e. 2%, and 0.01, i.e. 1%, respectively (ordinate) and specificity (abscissa)=1.00, i.e. 100%.
The outcome of OFC was not correlated: (a) with the type of signs or symptoms characterizing the ARE, except for the vomiting (OR=1.57, 95% CI=1.10–2.25, p=0.013); (b) with the comorbidity, except with the atopic dermatitis (OR=2.02, 95% CI=1.09–3.73, p=0.025); (c) with the age of the patient at the time of the 1st ARE; (d) with the latency time between ingestion of IF and the appearance of ARE; (e) with the number of previous innocuous ingestion of the IF; (f) with the number of organs involved in the ARE (mono-organ ARE vs multi-organ ARE); (g) with the time between the last ARE and OFC up to a maximum of 12 months.
The results of the remaining subgroup analysis were not performed due to the low number of the sample, such as the correlation between OFC outcome and: (a) severity of the symptom or sign; (b) type of IF and its possible processing; (c) amount of IF.
DiscussionMost definitions of “convincing” or “suggestive” history3,7,10 do not explicitly take into account the number of ARE as a parameter able to affect the PPV of a history compatible with the suspect of IgE-FA. Except in the case of Stiefel et al.9 who wrote: “Clinical history is the cornerstone of making a diagnosis. The history of two adverse reactions to peanuts has an 80% pretest probability of the child having a peanut allergy”. Unfortunately, the authors9 do not mention a scientific study to support this statement.
The quantification of the predictive value of the history has already been studied in recent years. For example, Cianferoni et al.10 developed a Food Challenge Score in order to predict the outcome of the OFC. The authors took into account: the size of the wheal of the prick test, the history of non-cutaneous adverse reaction, and the patient's age. A score of 3–4 was predictive of anaphylaxis following the OFC and in particular the PPV was: 62% for cow's milk, 92% for egg and 86% for peanut. DunnGalvin et al.11 examined the predictive capacity of six clinical factors (skin prick test, serum specific IgE, total IgE minus serum specific IgE, symptoms, sex, and age) and reported that 97% of cases were accurately predicted as positive and 94% as negative.
In no study, to our knowledge, the PPV of symptom reproducibility, which is the number of ARE, has been specifically studied. It is certainly intuitive that if the ARE is repeated several times, it is more likely that the IgE-FA is true. It is also probable that a great number of allergists would be inclined to avoid OFC if the parents of an infant reported, for example, that their child just presented even only two episodes of urticaria following the ingestion of cow's milk. The propensity to avoid OFC is directly proportional to the number of ARE, but to date the PPV of a certain number of AREs is not known, nor consequently which number of ARE has a sufficient PPV to avoid the OFC.
According to our results, the number of ARE is an important predictor of the definitive diagnosis, although perhaps less than one might think. Among the children who presented four ARE, for example, only 50% failed the OFC and the PPV of this ARE's number is “only” 86%. To obtain a really relevant PPV you need to get at least five ARE (PPV=100%). It is quite probable that this result suffers from the bias determined by the retrospective nature of our study, which has conditioned above all a low sample size, and it is therefore not possible to consider it conclusive. Another important variable is the definition of failed OFC and passed OFC. We referred to the joined document about OFC of the two leading US and European allergology societies.7 If the symptoms are mild, identified in this document with the green color, the OFC should not be considered positive (i.e. failed). This, naturally, has consequences on the results. For example, one of the two patients in the group with four ARE with passed OFC had mild rhinitis symptoms, falling into the “green” category, which appeared at the first doses of OFC and disappeared in the rest of the procedure. The same mild symptoms then appeared during the first days of home intake, after the OFC, to disappear permanently after the first week. If other investigators judged these symptoms sufficient to declare the OFC failed (positive), the results would change in the sense of a stronger predictive correlation between the number of ARE and the outcome of the OFC. The percentage of failed OFC we found in this study (52%) is similar to the usual percentage in the participating allergy centers. Akuete et al.12 reported that the rate of allergic reactions, after clinical low-risk OFC in a non-research setting, was 14% (95% CI, 13%–16%, range 13%–33%) across multiple centers in the United States. It is probable that the difference with our result can be justified by the fact that we have not enrolled only children with a low risk.
It is likely that the number of ARE with a PPV high enough to avoid OFC will be reduced if associated with certain signs or symptoms. For example, in our study, if the number of episodes of vomiting is ≥4, specificity and PPV are =100%: this result however came from the observation of only four children with this characteristic (each with four ARE characterized by vomiting). Because of the small sample size in the individual subgroups of signs and symptoms, we did not find it useful to expose the results of other signs or symptoms in detail, for which, however, the PPV was almost never higher than 90%. Another example of the combination “ARE+sign/symptom” was found in the group of children enrolled with the diagnosis of cow's milk allergy, where we observed that the PPV of the average diameter of PbP with pasteurized cow's milk estimated at 9mm passes from 95% to 100% if the number of ARE is ≥3, always proving that the number of ARE is an important component in the “suggestiveness” of the clinical history (data not shown).
Therefore, as already done by other authors10,11 with other parameters, the number of ARE could be used to increase the predictability of the diagnostic tests currently in use, to define clinical prediction rules alternative to OFC and easy to use in the clinical practice.
Author contributionsStefano Miceli Sopo and Giovanna Gurnari conceived the design of the study and drafting the article. Lucia Liotti, Barbara Cuomo, Iride Dello Iacono, Laura Badina, Giorgio Longo, Mauro Calvani, Andrea Giannone, Claudia Calabrò, Guglielmo Scala acquired the data. Alberto Romano and Maria Carmela Verga analyzed the data and interpreted them. Serena Monaco revised the article. All authors given final approval of the version to be published.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors have no conflict of interest to declare.