Early diagnosis and appropriate therapy are essential for the best prognosis and quality of life in patients with primary immunodeficiency diseases (PIDDs). Experts from several Latin American countries have been meeting on a regular basis as part of an ongoing effort to improve the diagnosis and treatment of PIDD in this region. Three programmes are in development that will expand education and training and improve access to testing facilities throughout Latin America. These programmes are: an educational outreach programme (The L-Project); an immunology fellowship programme; and the establishment of a laboratory network to expand access to testing facilities. This report provides the status of these programmes based on the most recent discussions and describes the next steps toward full implementation of these programmes.
Primary immunodeficiency disease (PIDD) is the name for a class of disorders in which the immune system fails to mount an adequate immune response as a result of intrinsic or genetic defects in the immune system1. The World Health Organization recognises more than 150 PIDDs2,3. Individuals affected with PIDD are at a higher risk of frequent, life-threatening, serious infections and have recurring infections. They also have a higher incidence of non-infectious complications such as malignancies, autoimmune diseases, and complications of the gastrointestinal tract (ie, malabsorption, inflammatory bowel disease, and gastritis)4. Untreated or inadequately treated PIDD can lead to long-term health problems, including permanent damage to organs such as the ears or lungs, or physical disability5, and can result in hospitalisations from infections, as well as lost days from work or school6. It is also associated with a substantial cost and resource-use burden7, with the largest cost driver being hospitalisation8.
Although PIDDs are generally considered to be rare, evidence indicates that they are more common than previously estimated9. It is thought that 70% to 90% of PIDDs remain undiagnosed10. Recent estimates of the rate of PIDD, as presented to the European Parliament11, indicate that:
- •
Approximately 1 in 8000 to 10,000 people has a genetic primary immunodeficiency that significantly affects their health.
- •
PIDDs affect at least 10 million people worldwide.
- •
The actual prevalence of some form of PIDD in the general population is estimated to be between 1 in 250 and 1 in 500. This compares with a prevalence of 1 in 700 for type 1 diabetes and 1 in 1000 for multiple sclerosis.
- •
While each of the more than 160 identified PIDDs may be rare, taken together, they are more common than childhood leukaemia and lymphoma combined.
Early diagnosis and appropriate therapy are essential for the best prognosis and quality of life in PIDD patients. Unfortunately a diagnosis of PIDD is often delayed1,12–15. Several factors may contribute to this delay, including a lack of awareness concerning these disorders and the variability of their clinical manifestation16,17.
Effective education of clinicians and enhanced public awareness of PIDDs has been shown to increase diagnosis and referral18,19. Moreover, diagnosis of patients leads to improved health, as evidenced by decreased rates of infection and reduced need for antibiotics or hospitalisation20.
Several international initiatives are currently ongoing to promote awareness of PIDD (http://www.primaryimmune.org/resources/resources.htm) and increase the diagnosis and registration of individuals with PIDD. Among these is the Latin American Society for Immunodeficiencies (LASID; formerly known as the Latin American Group for Immunodeficiencies [LAGID]). LAGID was established in 1993 to study the prevalence of PIDDs in the diverse regions of Latin America and to promote awareness of these diseases. Since its inception, LAGID has issued two reports21,22 describing the characteristics of PIDD patients from Latin America. An online registry has also been established (http://deficiencia.unicamp.br:8080/) to provide further information about the epidemiology of PIDDs in Latin America.
A variety of factors negatively impact the level of diagnosis and management for Latin American patients with PIDD. Most important is the fact that many Latin American paediatricians and general practitioners are not sufficiently trained to suspect PIDD, and as a result, there can be a substantial delay in diagnosis and treatment. Other factors that complicate and delay diagnosis and treatment include: limited availability of specialised testing facilities, limited coverage for screening tests by both governmental and private health plans, regional variability of access to intravenous immunoglobulin (IVIG) treatment, and the absence of IVIG treatment guidelines in many countries as well as failure to adhere to established guidelines in some regions. Recently, experts from six Latin American countries and the United States met to discuss three programmes aimed at improving the diagnosis and management of PIDDs in Latin America (the Latin America Advisory Board on Primary Immunodeficiencies): an educational outreach programme (The L-Project), an immunology fellowship programme, and the establishment of a laboratory network to expand access to testing facilities. This report summarises these discussions.
Educational outreach-The L-ProjectThe objectives of the L-Project are to educate patients and families, to enhance the quality of the education concerning PIDDs, to promote basic and clinical research and study, and to inform governments about the public-health impact of PIDDs, while increasing the awareness of the general public. Unlike other educational programmes18 that focus only on primary care clinicians, the target audience for the L-Project includes medical students, paediatric residents, fellows, practicing generalists, internists, paediatricians, subspecialists (eg, rheumatologists, pulmonologists, and neonatologists), and nurses, as well as all levels of health authorities and the general public. An essential goal of the L-Project is not only to train clinicians, but also to encourage medical students to aspire to academic positions in teaching and research in the area of PIDD.
Critical features of the L-Project are repetition and reinforcement. Educational programmes will be developed for each of the target groups and will utilise activities appropriate to their needs and knowledge level. Examples of programmes include summer courses devoted exclusively to immunodeficiencies; a series of case-based interactive lectures and short programmes focused on infections associated with PIDD; educational material for adults, children, and parents of children diagnosed with PIDD that addresses their specific concerns and questions; and radio, newspaper, magazine, and television advertisements to inform the general public.
Many of the materials to be used in the L-Project already exist; what remains is for them to be adapted for targeted groups and for the programmes to launch. However, resources need to be identified and committed to staff the programmes. The Advisory Board discussed the establishment of a PIDD Community Network - an online community of healthcare professionals capable of providing education, clinical consulting, and professional interaction on a peer-to-peer basis. The online programme would serve as a “clearing station” for information about PIDD; it would provide for one-to-one communication among clinicians; make intensive training courses available; and allow access to videos, direct links to other PIDD-oriented Web sites, and downloadable handouts (eg, from prior lectures or for distribution to patients). The site would also contain information about ongoing research projects. One of the training programmes being considered for the Web site is a case-based learning course. Enrolled medical students, residents, and fellows will be asked to discuss cases posted by experts in the field. Their discussions will be moderated and participation evaluated. A grade will be issued at the end of each section. Each course will comprise of several cases and a certain level of achievement will be needed to progress to the next course. Ultimately, the online site would function similarly to many of the social-media sites (eg, Twitter or Facebook), but its focus would be on scientific communication. The framework for such an online system is already in place.
The fellowship programmeFellowships, whether funded by institutions, governments, or private entities, are commonly used to support the development of individuals who have demonstrated a commitment to study in a particular area as a career path. In medicine, this path may be as a practicing clinician or in academic research. After much discussion and in recognition of the differing needs among the represented countries, the Advisory Board determined that fellowships should be available to clinicians interested in clinical practice in immunology, as well as clinicians and/or PhD's interested in immunology research. The goal is to establish several two-year, non-renewable fellowships administered by a committee of LASID members and open only to LASID members. Institutions wanting to participate must agree to participate in the LASID PIDD Registry by registering a percentage of their patients. Although specific eligibility criteria will be established by LASID, typical information requirements include: a personal statement from the applicant, their career development plan, a description of their current training and research environment, an outline of the applicants career development project, an applicant biography, identification of a primary mentor, statement of any financial conflict of interest, and letters of recommendation. It was agreed that no single country could be awarded more than two fellowships during any two-year period. Recipients will be required to publish a report of their clinical activities or the results of their clinical research. Fellowship applications will be accepted during, and winners announced, at the biannual LASID meeting. One person from LASID would be selected as the primary contact for questions, receipt of applications, etc.
Diagnosis of PIDDThe Jeffrey Modell Foundation (JMF) has developed a list of 10 warning signs which can be used as an aid to diagnosing PIDD, including ≥4 ear infections within one year, ≥2 serious sinus infections within one year, ≥2 months on antibiotics with little effect, failure of an infant to gain weight or grow normally, recurrent, deep-skin or organ abscesses, persistent thrush in mouth or fungal infection on the skin, need for intravenous antibiotics to clear infections, ≥2 deep-seated infections including septicaemia, and a family history of PIDD. The presence of ≥2 of these signs may indicate PIDD23.
Since most PIDDs are lifelong conditions, it is important to perform a detailed diagnostic evaluation before beginning therapy; thus, once PIDD is suspected, patients should be referred to an immunologist to confirm the diagnosis and initiate a treatment plan. Four stages of testing, including history and physical examination and testing for numerous specified humoral, cellular, complement, and molecular indicators are recommended for PIDD diagnosis23.
Laboratory networkAlthough many Latin American laboratories are capable of performing some of the recommended tests, access to laboratory testing in Latin America is generally limited. Budgetary restrictions often mean that not all tests can be performed at all laboratories, and even when testing is available, the cost is not always covered. This is particularly true for some of the more specialised tests. A limiting factor for the expansion of testing facilities in many institutions is funding. Most laboratories are underfunded, particularly for staff salaries and the purchase of reagents necessary for some tests. Currently, the major sources of funding for the laboratories are firstly the hospital or institution in which they are housed; and secondly, grants associated with research projects. However, several laboratories have successfully adopted a “fee-for-service” system whereby outside or private institutions are charged for testing services, and the income is used to support the laboratory and cover the cost for individuals who are unable to pay for their own testing. Cost reduction is another option being considered as a way to make possible expanded laboratory services.
To compensate for the uneven availability of testing facilities, many of the laboratories have been collaborating for several years. However, results of a recent survey which investigated diagnostic practices at some of the laboratories showed that practices varied substantially among institutions. The survey was conducted at 11 medical centres in six different Latin American countries (two in Argentina, three each in Brazil and Chile, and one each in Colombia, Costa Rica, and Mexico). The laboratories were asked to provide data on the type of tests performed, geographical area served, billing practices, specimen handling and shipping practices, whether the laboratory had a PIDD serum and DNA bank, and whether it wished to take part in a laboratory network.
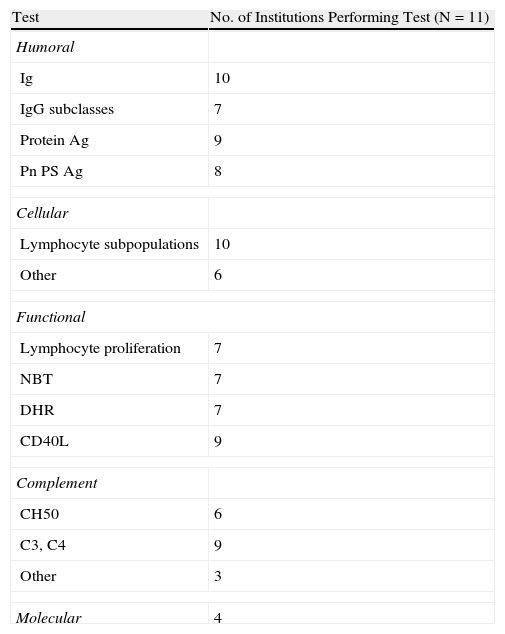
Of the JMF-recommended diagnostic tests, 10 of the 11 laboratories tested immunoglobulin (Ig) levels or lymphocyte subpopulations. Nine of the institutions tested for antibodies to protein antigens and eight to pneumococcal polysaccharides. CD40L deficiency, and C3, C4 complement fractions were tested in nine centres. Only seven laboratories tested for IgG subclasses, lymphocyte proliferation, and oxidative burst by the nitroblue tetrazolium (NBT) or the dihydrorhodamine (DHR) methods; six performed CH50 complement screening; four conducted molecular tests; and three performed complement testing beyond C3, C4, and CH50 (Table 1).
Tests Performed at Surveyed Institutionsa
| Test | No. of Institutions Performing Test (N=11) |
| Humoral | |
| Ig | 10 |
| IgG subclasses | 7 |
| Protein Ag | 9 |
| Pn PS Ag | 8 |
| Cellular | |
| Lymphocyte subpopulations | 10 |
| Other | 6 |
| Functional | |
| Lymphocyte proliferation | 7 |
| NBT | 7 |
| DHR | 7 |
| CD40L | 9 |
| Complement | |
| CH50 | 6 |
| C3, C4 | 9 |
| Other | 3 |
| Molecular | 4 |
Ag=antigens; DHR=dihydrorhodamine; Ig=immunoglobulin; NBT=nitroblue tetrazolium; Pn Ps=pneumococcal polysaccharide.
Eight laboratories reported having specimen-handling and quality-control requirements; five had a serum bank, and seven had a DNA bank from PIDD patients. Half of the laboratories charged for tests, one for out-of-country requests only. Six laboratories served their entire country, and five served other countries; however, six reported problems with shipping samples to other countries. Seven laboratories expressed willingness to take part in a Latin American laboratory network.
The substantial variation in practices among the laboratories indicates that a more formal network is needed to facilitate best practices in PIDD diagnosis and management. The goals of the LASID Laboratory Network are to establish a laboratory network for specialised tests; implement proficiency and quality-control programs; offer training on specialised testing to other laboratories; and to publish the list of participating laboratories along with the tests they can perform, specimen requirements, and methods for specialised tests; and establish collaborative research studies. The group is also pursuing the possibility of a reduction in the cost of reagents by negotiating improved pricing with the reagent suppliers and establishing a longer-term goal of having at least one or two laboratories with a repository of DNA samples with known mutations to support neonatal screening.
SummaryBecause of their differing healthcare systems, it is not feasible to develop a single programme for all of Latin America. Regional- and/or country-specific programmes that take into account the differences in educational needs, access to treatment, reimbursement policies, and governmental regulations are required. The programmes discussed in this report are being developed with such considerations in mind, and along with the Latin American PIDD Registry, will improve the diagnosis and management of PIDD. As a final step in the development of the L-Project, the group is conducting a survey of all LASID members.
Conflict of interestJosé Luis Franco has received consulting fees from Baxter. All other authors declare no conflicts of interest.
The authors thank Baxter Biosciences for supporting the Latin American Advisory Board on Primary Immunodeficiencies initiative, as well as supporting the editorial services for the development of the proceedings of this initiative.