Omalizumab is indicated in patients with severe allergic asthma not controlled by high-dose inhaled glucocorticoids and long-acting beta-agonists. Few data are available on the profile of patients treated with this drug in routine clinical practice in Spain.
ObjectiveTo describe the profile of patients with severe allergic asthma treated with omalizumab and the course of the disease after a period of treatment.
MethodsRetrospective, multicentre study, recording the data on patients of either sex and ≥12 years with uncontrolled severe allergic asthma, previously treated with omalizumab. Data were evaluated in relation to pulmonary function, symptoms, quality of life, and concomitant anti-asthma treatment before the prescription of omalizumab and at the time of the study visit.
Results214 patients were evaluable (mean age=48.2±17.7 years; mean age at the time of diagnosis=26.6±16.5 years). 90.7% had experienced exacerbations the year before receiving omalizumab, and the mean total IgE level was 273±205.4IU/ml. The mean monthly dose was 380.5±185.4mg. Compared with the baseline situation, differences were observed after treatment with omalizumab in mean FEV1 (62.7±15.9% vs. 70.8±18.7%), in the proportion of patients requiring oral corticosteroids (47.7% vs. 14.0%), and in the ACQ and AQLQ scores. 32.7% of the patients received doses not recommended by the Summary of Product Characteristics (SPC).
ConclusionsProfile of asthmatic patients treated with omalizumab predominantly corresponds to uncontrolled severe asthma cases, in accordance with SPC's indications. The results of the study suggest a favourable clinical course similar to that observed in other studies.
Asthma is a chronic pulmonary inflammatory disease affecting approximately 300 million people worldwide.1 The mortality risk in asthmatic patients increases with the clinical severity of the condition – the latter being a predictor of mortality independently of the number of admissions to hospital.2,3
The Global Initiative for Asthma (GINA) 20084 guidelines recommend stepwise therapy according to the severity of the disease, taking into account that for good disease control, special emphasis on the prevention of exacerbations, hospitalisations and symptoms is required.
Despite the available treatments and recommendations of the different guidelines, many asthma patients are inadequately controlled,5–8 even after receiving high doses of inhaled corticosteroids in combination with other antiasthma drugs. In Spain, approximately 70% of all asthmatic patients are poorly controlled, and 10% are totally uncontrolled, according to the results of the Study of the Control of Asthma in Spain (ESCASE).9 A recent European study10 has shown that approximately 50% of all patients with severe asthma had not reached the treatment objectives recommended by the GINA.4
Considering the inadequate control of asthma, and knowing that approximately 50–80% of all patients with severe asthma present an immunoglobulin E (IgE)-mediated allergic component that modulates the cascade triggering the allergic inflammatory reaction,10–12 omalizumab was developed as a monoclonal antibody. It binds to circulating IgE (anti-IgE), inhibiting the binding of IgE to Fc¿RI on mast cells and basophiles by binding to an antigenic epitope on IgE that overlaps with the site to which Fc¿RI binds, preventing the mentioned inflammatory reaction.13 Different clinical trials have demonstrated the efficacy of omalizumab,14–19 with a reduction in the number of exacerbations and hospital admissions, as well as improvement in the quality of life of patients with persistent moderate or severe asthma. Accordingly, the GINA 20084 guidelines include treatment with omalizumab as an adjuvant to inhaled corticosteroids plus long-acting β2-agonists.Due to the recently obtained marketing authorisation in Spain, there are not enough data to establish the characteristics of the patients receiving treatment with omalizumab and its use in routine clinical practice – the existing information being limited to a few case series.20–22
Thus, the present study was designed to describe the profile of patients with severe allergic asthma treated with omalizumab in routine clinical practice, and to establish the course of the disease after a period of time receiving the drug.
Materials and methodsStudy design and populationThe RIGE study is a retrospective, multicentre study involving the participation of 60 specialists in pneumology and allergology from all over Spain.
During five months, data were collected on patients of both sexes and aged 12 years or older, who had received at least one dose of omalizumab. Another inclusion criterion was that patients had to be included according to SPC. In all cases, written informed consent was obtained from the patient or legal representative or tutor (in the case of minors). Patients who had participated in a clinical trial with omalizumab or other anti-asthma drugs in the year prior to inclusion were excluded, as well as those patients who had a situation that limited their participation in the study. A sample size of 221 patients provided a precision of ±5.0% to estimate the factors determining the continuity of treatment with omalizumab in patients with severe allergic asthma, with a 95% confidence interval (95% CI). Assuming 10% of patients would not be valid for the analysis, the number of patients to be recruited was 246.
The study was approved by the Ethics Committee of the Barcelona Clinic Hospital, Barcelona, Spain and notified to the Spanish Agency for Medicines and Medical Devices (AEMPS). Written informed consent was obtained from all patients before their entry into the study.
Data collectionThe following patient information was collected from the medical records on a retrospective basis: biodemographic data (age, sex, weight, height), clinical history of asthma (personal and family disease antecedents, date of diagnosis, presence of exacerbations), data related to treatment with omalizumab (dose, frequency and duration of treatment), IgE levels and pneumoallergen skin prick tests.
To evaluate the patient's course, data corresponding to the period before the prescription of omalizumab and at the time of the study visit were collected, as well as information on pulmonary function (forced vital capacity (FVC), forced expiratory volume in one second (FEV1) and peak expiratory flow (PEF)), concomitant drug treatment for asthma, symptomatology (symptoms, Asthma Control Questionnaire (ACQ) score),23 and quality of life (Asthma Quality of Life Questionnaire (AQLQ) score).24 In addition, an evaluation was made of the degree to which patient life was affected by the disease, according to investigator criterion (rated as not at all, a little, quite a lot, or a lot). A global evaluation was also made of the efficacy of the treatment, based on the Global Evaluation of Treatment Efficacy (GETE). For the patients who discontinued treatment with omalizumab prior to their participation in the study, the post-treatment information considered was that at the time of withdrawal.
Treatment with omalizumabStudy was done on whether the patients included in the study had been prescribed the treatment according to the indications of the SPC of omalizumab, was evaluated based on a positive skin prick test, the presence of diurnal and nocturnal symptoms, whether concomitant therapy was provided with inhaled corticosteroids and β2-agonists, and the FEV1. Study was also done on whether the dose level received corresponded to the doses recommended by SPC, which is defined by IgE levels and body weight.
Statistical analysisCategorical variables were reported as absolute and relative frequencies, while continuous variables were expressed as the mean, standard deviation (SD), median, and minimum and maximum, including the total number of valid values.
The tests used to compare variables were chosen according to their characteristics. Parametric (Student t-test or analysis of variance (ANOVA)) or non-parametric tests were used for comparing quantitative variables (Mann–Whitney or Kruskal–Wallis test), as applicable in each case. The chi-square test in turn was used for comparing qualitative variables. All the statistical analyses were performed with a statistical package (SAS, Version 9.1.3).
ResultsDescription of the sampleA total of 230 patients were enrolled in this study of whom 16 did not meet the criteria or did not have data on the primary outcome; thus a final total of 214 patients were evaluable for the analysis. It was observed that 12.8% of patients showed a FEV>80%, despite that the investigator indicated that they met the SPC criteria.
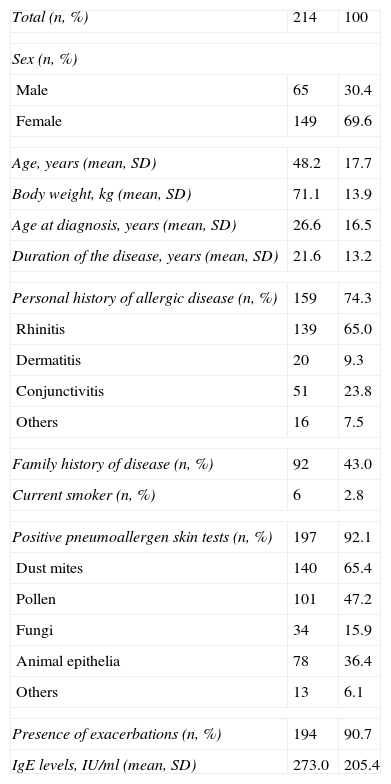
The majority were females (69.6%), and the mean age was 48.2 years (SD=17.7). At the time of asthma diagnosis, the mean age was 26.6 years (SD=16.5), with a mean time to disease progression of 21.6 years (SD=13.2). On the other hand, 74.3% of the patients had a personal history of allergy – rhinitis being the most frequent disease (65.0%) – and 90.7% had experienced exacerbations during the year prior to the prescription of omalizumab. A total of 92.1% of patients showed positive pneumoallergen skin prick tests, and 87.2% showed FEV1 <80%. The biodemographic characteristics and principal data relating to the clinical history of the patients are described in Table 1.
Biodemographic characteristics and clinical history of the patients.
| Total (n, %) | 214 | 100 |
| Sex (n, %) | ||
| Male | 65 | 30.4 |
| Female | 149 | 69.6 |
| Age, years (mean, SD) | 48.2 | 17.7 |
| Body weight, kg (mean, SD) | 71.1 | 13.9 |
| Age at diagnosis, years (mean, SD) | 26.6 | 16.5 |
| Duration of the disease, years (mean, SD) | 21.6 | 13.2 |
| Personal history of allergic disease (n, %) | 159 | 74.3 |
| Rhinitis | 139 | 65.0 |
| Dermatitis | 20 | 9.3 |
| Conjunctivitis | 51 | 23.8 |
| Others | 16 | 7.5 |
| Family history of disease (n, %) | 92 | 43.0 |
| Current smoker (n, %) | 6 | 2.8 |
| Positive pneumoallergen skin tests (n, %) | 197 | 92.1 |
| Dust mites | 140 | 65.4 |
| Pollen | 101 | 47.2 |
| Fungi | 34 | 15.9 |
| Animal epithelia | 78 | 36.4 |
| Others | 13 | 6.1 |
| Presence of exacerbations (n, %) | 194 | 90.7 |
| IgE levels, IU/ml (mean, SD) | 273.0 | 205.4 |
SD, standard deviation; IgE, immunoglobulin E.
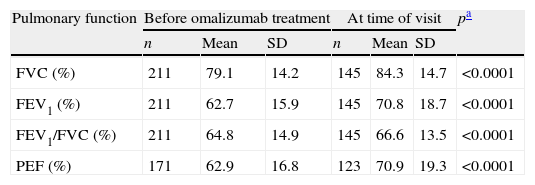
In relation to pulmonary function, a statistically significant increase was observed in all the parameters evaluated (FVC, FEV1, PEF and FEV1/FVC index) at the time of the visit with respect to the values prior to the prescription of omalizumab (Wilcoxon test; p<0.05) (Table 2). The most frequent concomitant anti-asthma treatments both prior to prescription and at the time of the visit were fixed-dose combinations of an inhaled glucocorticoid and a long-acting β2-agonist, leukotriene antagonists, and β2-agonists – a statistically significant decrease being observed in the percentage of patients receiving these treatments at the time of the visit (McNemar test; p<0.05) (Table 2).
Evolution of pulmonary function and concomitant anti-asthma treatments.
| Pulmonary function | Before omalizumab treatment | At time of visit | pa | ||||
| n | Mean | SD | n | Mean | SD | ||
| FVC (%) | 211 | 79.1 | 14.2 | 145 | 84.3 | 14.7 | <0.0001 |
| FEV1 (%) | 211 | 62.7 | 15.9 | 145 | 70.8 | 18.7 | <0.0001 |
| FEV1/FVC (%) | 211 | 64.8 | 14.9 | 145 | 66.6 | 13.5 | <0.0001 |
| PEF (%) | 171 | 62.9 | 16.8 | 123 | 70.9 | 19.3 | <0.0001 |
| Drug treatment | n | %b | n | %b | pc |
| Budesonide+Formoterol | 59 | 27.6 | 38 | 17.8 | <0.0001 |
| Fluticasone+Salmeterol | 140 | 65.4 | 94 | 43.9 | <0.0001 |
| Inhaled corticosteroids | 36 | 16.8 | 20 | 9.3 | <0.0001 |
| Oral corticosteroids | 102 | 47.7 | 30 | 14.0 | <0.0001 |
| β2-agonists | 145 | 67.8 | 80 | 37.4 | <0.0001 |
| Leukotriene antagonists | 164 | 76.6 | 98 | 45.8 | <0.0001 |
| Long-acting theophyllines | 30 | 14.0 | 12 | 5.6 | <0.0001 |
| Others | 76 | 35.5 | 29 | 13.6 | <0.0001 |
FEV1, forced expiratory volume in one second; FVC, forced vital capacity; PEF, peak expiratory flow.
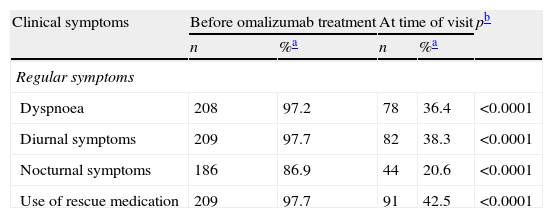
The evolution of the common symptoms of asthma (dyspnoea, diurnal symptoms, nocturnal symptoms), as well as the use of rescue medication showed a statistically significant decrease after treatment with omalizumab (McNemar test; p<0.05) (Table 3). The investigator considered the asthma symptoms to affect patient quality of life quite a lot or a lot in all cases (100.0%) before treatment with omalizumab, and in 33.6% of the cases at the time of the visit. The results of the ACQ questionnaire were only available for 25.7% of the patients before treatment and for 18.2% at the time of the visit. In the case of the AQLQ questionnaire, these percentages were 11.7% and 7.9%, respectively. Despite the small sample size, improvement was observed in patient symptoms and quality of life based on the mean scores of the ACQ and AQLQ questionnaires (Wilcoxon test; p<0.05) (Table 3).
Evolution of the clinical symptoms and quality of life.
| Clinical symptoms | Before omalizumab treatment | At time of visit | pb | ||
| n | %a | n | %a | ||
| Regular symptoms | |||||
| Dyspnoea | 208 | 97.2 | 78 | 36.4 | <0.0001 |
| Diurnal symptoms | 209 | 97.7 | 82 | 38.3 | <0.0001 |
| Nocturnal symptoms | 186 | 86.9 | 44 | 20.6 | <0.0001 |
| Use of rescue medication | 209 | 97.7 | 91 | 42.5 | <0.0001 |
| Quality of life | n | %a | n | %a | pc |
| What degree do you consider the asthma symptoms to affect patient quality of life? | 214 | 100.0 | 151 | 70.6 | <0.0001 |
| Not at all | 0 | 0.0 | 11 | 5.1 | |
| A little | 0 | 0.0 | 68 | 31.8 | |
| Quite a lot | 88 | 41.1 | 60 | 28.0 | |
| A lot | 126 | 58.9 | 12 | 5.6 |
| ACQ/AQLQ | n | Mean (SD) | n | Mean (SD) | pd |
| ACQ score | 55 | 3.0 (1.3) | 39 | 2.5 (1.7) | <0.0008 |
| AQLQ score | 25 | 3.1 (1.0) | 17 | 4.8 (1.4) | <0.0010 |
SD, standard deviation.
10.4% of patients considered Global Evaluation of Treatment Efficacy (GETE) to be excellent, 56.5% good, 27.5% moderate, and only 5.7% reported no appreciable changes.
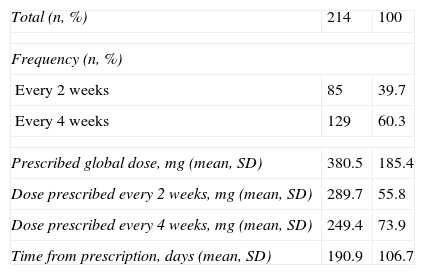
Treatment with omalizumabIn reference to treatment with omalizumab, the mean dose was found to be 380.5mg (SD=185.4), and 60.3% of the patients received the medication every four weeks (Table 4).
Treatment with omalizumab.
| Total (n, %) | 214 | 100 |
| Frequency (n, %) | ||
| Every 2 weeks | 85 | 39.7 |
| Every 4 weeks | 129 | 60.3 |
| Prescribed global dose, mg (mean, SD) | 380.5 | 185.4 |
| Dose prescribed every 2 weeks, mg (mean, SD) | 289.7 | 55.8 |
| Dose prescribed every 4 weeks, mg (mean, SD) | 249.4 | 73.9 |
| Time from prescription, days (mean, SD) | 190.9 | 106.7 |
SD, standard deviation.
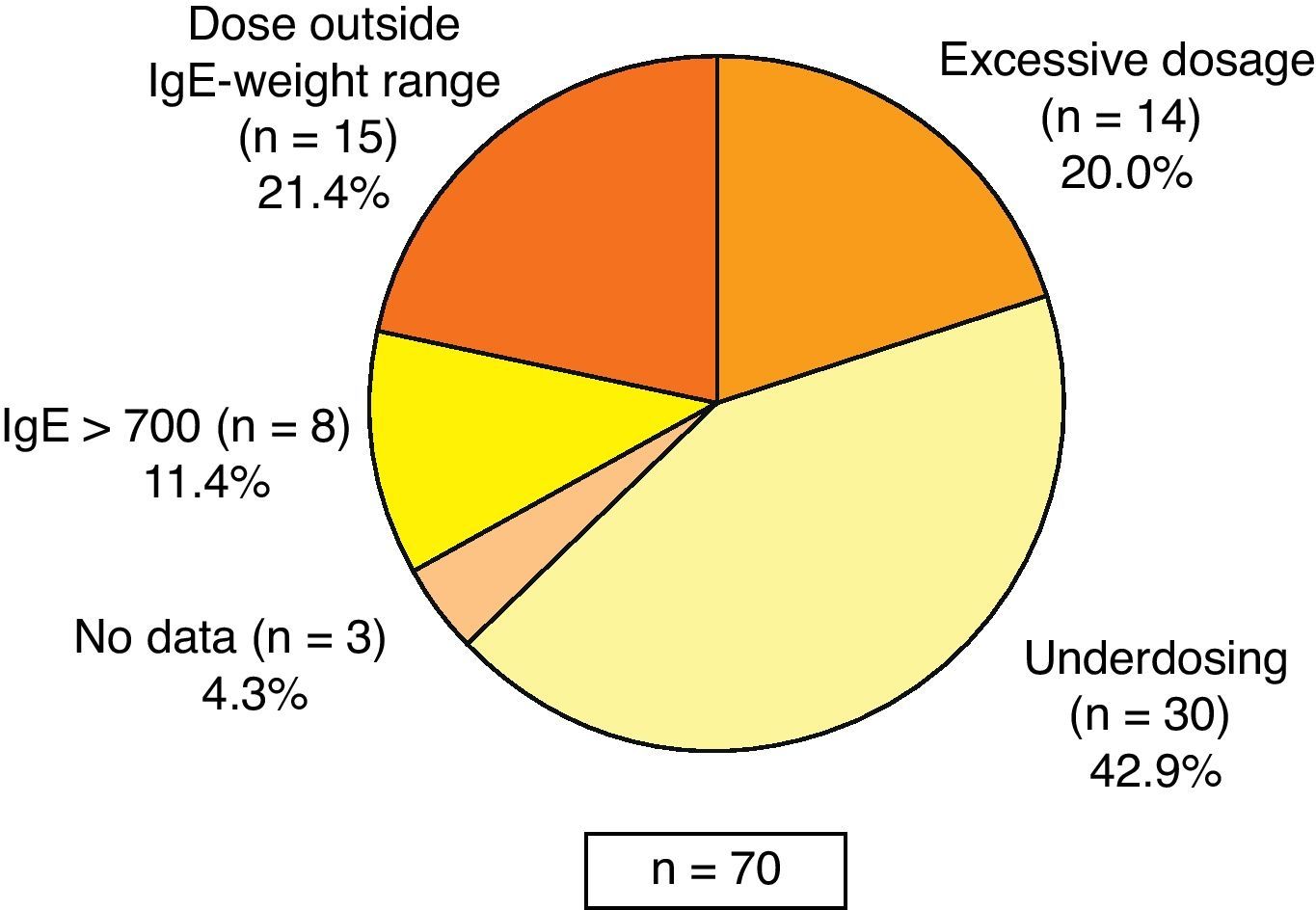
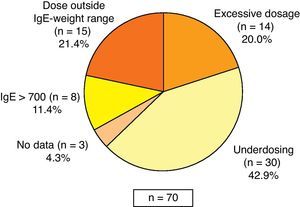
Most of the patients received omalizumab treatment according to the indications of the SPC, although 12.8% of the patients showed no reduction in lung function (FEV1) below 80%. In reference to the prescribed dose, it was observed that 32.7% of patients received doses outside those specified in the SPC: 42.9% of those receiving less than the recommended dose (of these, 70.0% received doses every four weeks instead of every two weeks), 21.4% of patients received the drug despite absence of dosing recommendations according to SPC (based on weight and IgE levels), 20.0% of patients received higher than recommended doses, 11.4% had baseline IgE levels above 700IU/mL and in 4.3% of cases no data were available (Fig. 1). Most of the patients (92.1%) continued with omalizumab at the time of the visit. The rest (7.9%) discontinued the treatment due to the following reasons: decision of the patient (4.2%), lack of treatment response (2.3%), and discomfort related with the treatment (1.9%).
DiscussionThe present study offers new data on the profile of patients with persistent severe allergic asthma treated with omalizumab in routine clinical practice in Spain. Statistically significant improvement has been observed in the spirometry results and in the symptoms (ACQ) and quality of life (AQLQ) questionnaires, and a reduction has been recorded in concomitant anti-asthma treatment compared with the time of initiating treatment.
A limitation of the study has been its retrospective design, which precluded information about the number of exacerbations or hospitalisations. In addition, by collecting the data before the prescription of omalizumab and at the time of the visit (retrospective), the variables used to assess the patient course (spirometry, concomitant anti-asthma treatment, symptoms and quality of life questionnaires) were not reported systematically, and information therefore was not available on all the patients. Another limitation of the study, also due to the retrospective design, is a possible selection bias of investigators choosing patients who had had a good response to treatment with omalizumab.
Despite these limitations, the results of the study are similar to those reported in other prospective studies in patients with persistent asthma in Spain20–22 and in other European countries (France, Germany and Belgium).25–27 The studies conducted in the setting of routine clinical practice have consistently yielded results even superior to those of controlled clinical trials.16,28
Moreover, taking into account the limited information available to date due to the recently obtained marketing authorisation in Spain makes the findings even more interesting, especially with respect to clinical characteristics of patients and compliance with the indications of the SPC.
The clinical studies with omalizumab29 have incorporated quality of life assessments based on validated questionnaires such as the AQLQ – patients treated with omalizumab showing significant improvement in all aspects related to quality of life. Thus, in evaluating asthma control, the recommendation is not only to collect clinical data but also to conduct patient follow-up with quality of life and symptoms questionnaires. In the study, follow-up with the AQLQ and ACQ questionnaires had only been carried out in 7.9% and 18.2% of the patients, respectively, so the above recommendations were not followed.
Most of the patients in our study had been prescribed omalizumab in compliance with the indications of the SPC. In addition, they were receiving concomitant drug treatment for asthma with the combination of inhaled glucocorticoids and long-acting β2-agonists, and 47.7% were using oral glucocorticoids. In spite of the limitations of this study design, to the knowledge of the authors, the data presented here are the first data available in Spain about the profile of these patients and are similar to that reported by the studies conducted in routine clinical practice in other countries such as France25 or Germany,26 while in Belgium27 the patient condition was somewhat more severe.
It was observed that 32.7% of patients received doses not recommended by the SPC. Many such doses were lower than those recommended (42.9%), in a proportion greater than that reported in other countries. In Spain the practice mainly consisted in the reduction of omalizumab administration from every 15 days to once a month. The present study reinforces the importance of the need to adhere to the dosing recommendations of the SPC, since inadequate dosing may limit the therapeutic effect of the treatment.30 On the other hand, some patients received the drug inappropriately (21.4%), since the tables for calculating the dosage are limited to certain IgE and body weight values. In the study, treatment discontinuation was lower than in the prospective studies25–27 (7.9% vs. over 30%). Such discontinuation may have been underestimated as a result of the study design, since the patients who discontinue treatment go to the visit less often and have fewer chances of being selected for the study.
Although no safety data were collected during the study, we found that of the 16 patients who discontinued treatment, only four did so because of problems associated with the medication. This suggests good tolerability of omalizumab, as has been demonstrated in different clinical trials.31
ConclusionsThe retrospective study with 214 Spanish patients treated with omalizumab indicates that prescription of the drug was mainly made in patients with severe allergic asthma, not controlled by high doses of inhaled glucocorticoids and long-acting beta-agonists, according to the indications of the SPC. Despite this, an important percentage of cases receiving doses lower than those recommended were reported. The results suggest that the patient course was comparable to that reported in prospective studies conducted in the setting of routine clinical practice. Patient follow-up based on quality of life and symptoms questionnaires has been much lower than recommended.
Ethical disclosuresProtection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Patients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis study was sponsored by Novartis Farmacéutica S.A. Barcelona, Spain.
Conflict of interestThis study was sponsored by Novartis Farmacéutica S.A. Barcelona, Spain.
The authors would like to thank the RIGE investigators group as well as the Medical Writing department of Adknoma for their assistance in the writing of this manuscript.