INTRODUCTION
In previous papers it was demonstrated that a extract of Triatoma infestans (Ti) a reduviid belonging to the Triatominae subfamily was capable to elicit a specific IgG response in rabbits, a specific IgE in atopics suffering perennial rhinitis and asthma and a hypersensitivity pneumonitis in an animal model1-4.
Peptidases are classified in serine, cysteine, aspartyl and metalloproteinases, according to: 1) the reaction that they catalyze; 2) the chemical nature of the catalytic site and 3) the evolutionary relationship revealed by their structures5.
In this paper, we present evidence indicating that Ti contain serine-proteinases with gelatinolytic properties which might be involved in their immunogenicity.
MATERIALS AND METHODS
Antigens
Ti and Periplaneta americana (Pa) were collected and both extracts were prepared as it was previously described following Frugoni-Hansen's method with slight modifications6. The Bradford method was applied to establish the protein content of Ti and Pa although this last antigen seems to be similar to that baptized as Per a 77.
Antisera
Adult albino rabbits were immunized against the whole Ti extract (13 mg/ml). Thus a rabbit polyclonal anti-Ti serum was obtained1. Human sera were collected from atopic patients suffering rhinitis/asthma who exhibited positive immediate skin tests and IgE-RAST ≥0.35 PRU/ml to Ti and to Pa separately.
Polyacrilamide gel electrophoresis
SDS-PAGE was performed by the method of Laemmli8 using a 4 % stacking gel and a 15 % running gel in a Mini-Protean II apparatus. Twenty microliters of Ti and Pa were loaded in separated wells with reducing and boiling conditions both for the detection of proteins by Coomasie R 250 brilliant blue and for electrotransference to a nitrocellulose membrane9.
Enzymatic activity assay
Minigels of 10 ×10 cm each and 1,5 mm thick composed of 12 % acrylamide were made as described by Laemmli () with gelatin at a final concentration of 0.2 %. They were run at 130 V for 2 hs. When the bromophenol blue used as a marker reached the bottom, the run was stopped and the gels were washed twice in distilled water with Triton-X-100, 0.15 % for 15 min each, then incubated at 37 °C in 0.1 % 2-[N-morpholino] ethane sulfonic acid (MES) buffer at pH 6, in Tris AcH 100 mM pH 3.5 and Tris ClH 100 mM pH 8.5 containing 0.5 mM dithiothreitol (DTT).
The reaction was stopped and the remaining protein was stained by incubation at room temperature with 0.25 Coomasie brilliant blue R-250 in methanol-acetic acid-water 5:1:5 (v/v/v).
After destaining in methanol 20 % and acetic acid 10 %, the active bands were observed as colorless over a blue background.
Inhibitory assays
The washing and incubation of the gels were done with and without the protease inhibitors. The solutions employed were E 64 (L-trans-epoxy-succinylleucylamido[4-guanidino] -butane) 20 μM; tosyl-lysyl-chloro-methyl-ketone (TLCK) 100 μM; tosyl-phenyl-alanyl-chloro-methyl-ketone (TPCK) 1 mM; phenyl-methyl-sulphonyl-fluoride (PMSF) 10 mM; leupeptin 100 μM; o-phenantroline 1 mM and pepstatin-A 2 μM.
The molecular weight markers were phosphorylase-b (97.4 kDa), bovine serum albumin (BSA) (66.2 kDa), ovoalbumin (45 kDa), carbonic anhydrase (31 kDa), trypsin inhibitor (21.5 kDa) and lysozyme (14.4 kDa). For activity gels, the samples were not reduced nor boiled before loading.
Western blots
The samples with or without β -mercaptoethanol and boiling were run in 15 % standard polyacrylamide gel in the presence of SDS (SDS-PAGE), electrotransfered to nitrocellulose sheets, blocked for 2 hs with a solution containing 2 % fatty acid-free BSA, 0.01 % v/v Tween-20, PBS pH 7.2 and then incubated overnight with rabbit polyclonal anti-Ti serum 1/400, human sera anti-Ti 1/10 and human sera anti-Pa 1/5. After overnight incubation with the rabbit or human antisera, respectively, and repeated washing the sheets were treated with 1/3000 goat anti-rabbit IgG horseradish peroxidase conjugate or 1/500 rabbit anti-human IgE specific for ∈-chains peroxidase conjugate at room temperature during 2 hs. The chromagenic detection was developed using α -chloronaphtol and hydrogen peroxide10.
RESULTS
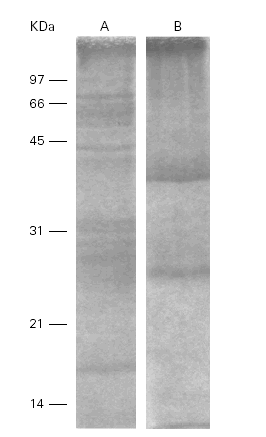
Ti in SDS-PAGE showed 10 to 12 bands between 14 and 100 kDa meanwhile Pa only showed 2 bands at 28 and 46 kDa. The total protein content of both extracts were 1 mg/ml (fig. 1).
Figure 1.--SDS-PAGE of the Ti (A) and Pa (B) extracts. Different bands are detected between 14 and 100 kDa in A and between 28 and 46 kDa in B.
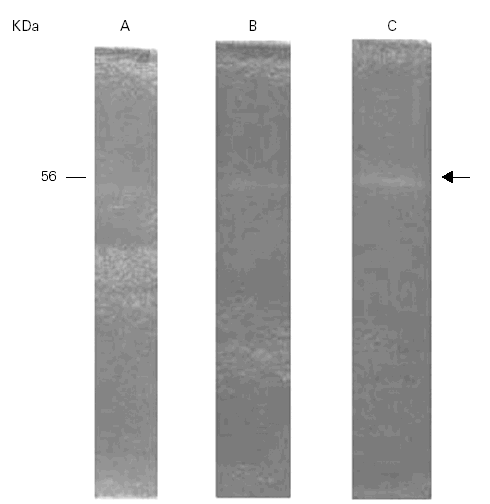
The gelatinolytic activity of Ti in SDS-PAGE with co-polymerized gelatin as substrate was recorded at 56 kDa. The proteolytic activity pattern of Ti was preliminary analyzed at three different pH levels, 4.5, 6.5 and 8.5. The highest enzyme activity was at pH 8.5 with less activity at 6.5 and 4,5 (fig. 2).
Figure 2.--Gelatinolytic activity of the Ti extract at different pHs. A: pH 4.5; B: pH 6.5; C: pH 8.5. The highest activity is seen at pH 8.5 within the band of 56 kDa.
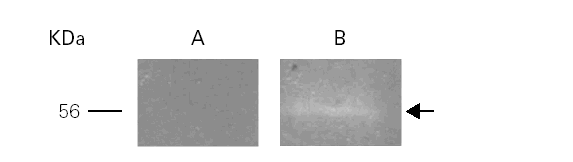
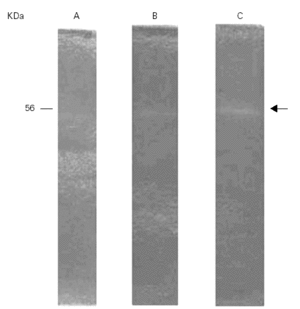
Total activity pattern at pH 8.5 was highly sensitive to PMSF exclusively suggesting that we can tentatively characterized this enzyme as a serine-protease (fig. 3).
Figure 3.--Inhibition of the gelatinolytic activity at 56 kDa with PMSF (A) and without PMSF (B).
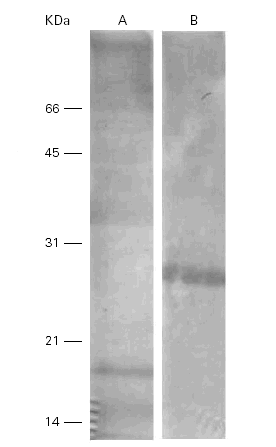
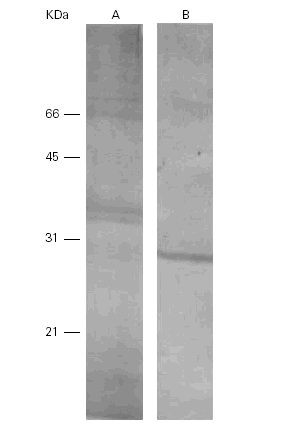
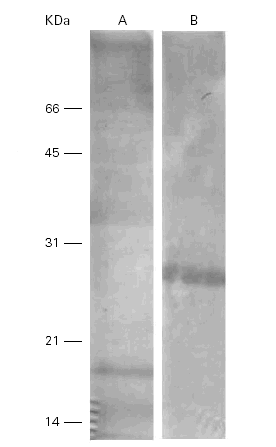
When the Ti was separated by SDS-PAGE, transferred to nitrocellulose and incubated with a polyclonal rabbit anti-Ti serum, the bands of 15 kDa, 18 kDa and 36 kDa showed immunoreactivity but only one band of 28 kDa cross-reacted with the Pa extract (fig. 4).
Figure 4.--Western-blots of the Ti (A) and Pa (B) incubated with a rabbit anti-Ti serum. Bands of 15, 18 and 36 kDa are seen in lane A and band of 28 kDa in lane B. Cross reactivities are seen among them.
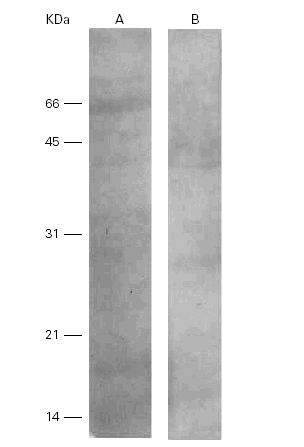
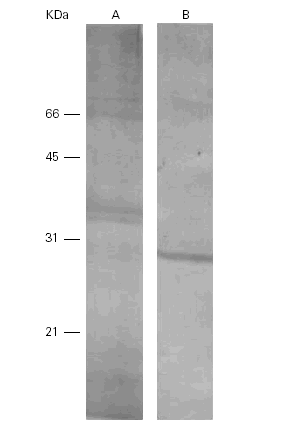
On the other hand, a human anti-Pa serum recognized a band of 28 kDa in Pa and cross-reacted with several bands in Ti (14 kDa, 35 kDa, 36 kDa and 75 kDa) (fig. 5).
Figure 5.--Western-blots of the Ti (A) and Pa (B) incubated with a human anti-Pa serum. Bands of 14, 35, 36 and 75 kDa are seen in lane A and a band of 28 kDa in lane B. Cross reactivities are seen among them.
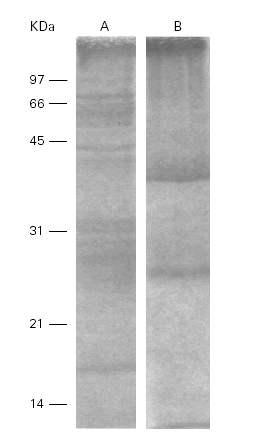
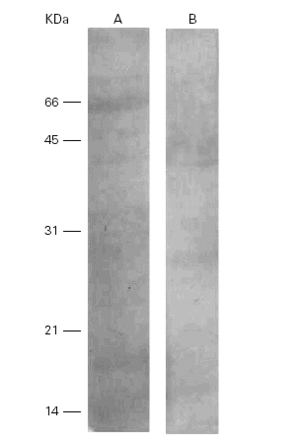
A human anti-Ti serum recognized 2 bands of 17 kDa and 58 kDa with its own antigen meanwhile in the cross-reaction with Pa these 2 bands were located at 28 kDa and 45 kDa (fig. 6).
Figure 6.--Western-blots of the Ti (A) and Pa (B) incubated with a human anti-Ti serum. Bands of 17 and 58 kDa are recognized in lane A and band s of 28 and 45 kDa are seen in lane B. Cross reactivity is evident among them.
DISCUSSION
Several major allergens in the extracts of insects possess protein hydrolases in their composition. Also, acid phosphatase activities correlate well with allergenic potency in pollen extracts.
Elegant studies showed the identification of a novel serin-protease with allergenic activity from Dermatophagoides pteronyssinus11.
In previous papers we described the correlation between some proteases with gelatinolytic activity and the allergenicity of house-dust mite, cockroach and bat feces extracts as it was revealed by specific Western blots12.
We suppose that this is the first report about the serine protease and gelatinolytic activities of the Ti extract although the relationship between these properties and its immunogenicity could not be clearly established.
Considering that many different proteins may be present in each broad band we cannot rule out the fact that the connexion would be possible if we take into account that the biological interactions between protease epitopes and the IgE or RcT receptors were demonstrated in other models.
It was also confirmed that the extracts of Ti and Pa exhibited common epitopes who were established in the past by our group.
It is very important to define the proteases present in all these extracts and to determine their ability to degrade other proteins of the same extract or in mixtures of allergen extracts.
Their role in the immunopathological aspects of rhinitis and asthma requires further investigation.