Parents’/caregivers’ quality of life is an important aspect to consider when handling paediatric asthma, but there is a paucity of valid and reliable instruments to measure it. The Family Impact of Childhood Bronchial Asthma (IFABI-R) is a recently developed questionnaire to facilitate the assessment of asthma-related parents’/caregivers’ quality of life. This study researches the psychometric properties of IFABI-R.
MethodsParents/main caregivers of 462 children between 4 and 14 years of age with active asthma were included in the sample. IFABI-R was administered on two different occasions and a number of other variables related to the parents’/caregivers’ quality of life were measured: child's asthma control, family functioning, and parents’/caregivers’ perception of asthma symptoms in the child. IFABI-R evaluative and discriminative properties were analysed, and the minimal important change in the IFABI-R score was identified.
ResultsIFABI-R showed high internal consistency (Cronbach's alpha=0.941), cross-sectional construct validity (correlation with the degree of child's asthma control, family functioning and parent/caregiver perception of the child's asthma symptoms), longitudinal construct validity (correlation of changes in the IFABI-R with changes in asthma control and changes in the perception of symptoms), sensitivity to change and test–retest reliability. An absolute change of 0.3 units in IFABI-R related to a minimal significant change in the parents’/caregivers’ quality of life.
ConclusionsIFABI-R is a reliable and valid instrument to study the quality of life of parents/caregivers of children with asthma.
Asthma is a chronic disease with a high prevalence in children: worldwide, 12–14% of school children and adolescents refer to having had recent asthma symptoms, according to the third phase of the International Study of Asthma and Allergies in Childhood1; similar figures have been observed in Spain.2 The current clinical guidelines for the management of patients with asthma establish that the therapeutic objective is to control the disease.3,4 This control has a clinical-based definition: symptoms, limitation of daily activities, need for rescue bronchodilators and alterations in pulmonary function tests. Numerous clinical trials have proven the effectiveness of the available medication to adequately control the disease; nevertheless, many children and adolescents with asthma have an insufficient degree of control.
Asthma affects patients’ quality of life, which is worse when the disease is not sufficiently controlled.5 However, the impact of asthma also affects the child's parents and caregivers, whose quality of life is closely linked to the degree to which the disease is controlled.6 This impact of asthma on the parents’/caregivers’ quality of life has attracted growing interest in research, as certain critical aspects of asthma management in children (such as therapeutic adherence) are closely linked to the way in which parents/caregivers perceive the burden of the disease on the child and themselves.7,8 Therefore, measuring the parents’/caregivers’ quality of life is important, both for research as well as for the clinical practice.
Measuring the quality of life of the parents’/caregivers’ of children with asthma requires having valid and reliable instruments. Until recently, there was only one specifically designed instrument: the Pediatric Asthma Caregiver's Quality of Life Questionnaire (PACQLQ).9 This instrument has been widely used in the literature since it was first published almost two decades ago; it has also been adapted to a variety of languages. There is a Spanish-language version that is used extensively in Spain and South America, but it has not been formally validated until now. Moreover, PACQLQ only researches two of the parents/caregivers’ quality of life spheres: physical-functional and emotional; its factorial structure lacks a specific domain that analyses the repercussion of childhood asthma in the social and labour life of their parents/caregivers, which is an extremely important aspect when evaluating quality of life.
In an effort to solve these limitations, a new instrument was recently developed to assess the quality of life of the parents/caregivers of children with asthma: the Family Impact of Childhood Bronchial Asthma-Revised (IFABI-R).10 Developed and validated in Spanish, this new instrument has proven to have an adequate factorial structure to assess the three main dimensions for the quality of life in parents/caregivers of children with asthma: the functional, emotional and socio-occupational dimensions. The initial study showed that the IFABI-R has an elevated internal consistency and construct validity verified by its cross-sectional correlation with measures of asthma severity, morbidity and the parents’/caregivers’ perception of symptoms.10 Moreover, the IFABI-R is currently being used successfully in clinical studies.6
However, it is essential to improve our knowledge of the psychometric properties of IFABI-R before it continues to be used clinically. Certain characteristics of this instrument—relating to validity, reliability and sensitivity to change—need to be defined further. The objective of this study is to expand the knowledge of the metric qualities of IFABI-R, through a new validation of the instrument.
MethodsSampleAt 22 primary care centres in Spain, the main caregivers of 4–14-year-old children with asthma were recruited. For inclusion, asthma had to have been diagnosed by a doctor and have shown symptoms within the previous 12 months. The accompanying adult identified him/herself as the child's main caregiver. Caregivers, who, in the opinion of the researchers, had a limited command of the Spanish language were excluded. The study was approved by the Ethical Committee at the centre coordinating the project (Palencia Healthcare Area, Spain) and the participants, both parents/caregivers as well as children granted written permission to take part in the study.
VariablesThe variables of the study were obtained through questionnaires filled out by the caregiver as well as by reviewing the patients’ clinical history. The variables measured were:
- (1)
The parents’/caregivers’ quality of life was studied using the Family Impact of Childhood Bronchial Asthma-Revised (IFABI-R),10 available after permission request in www.bibliopro.org. It is an assessment instrument that measures the quality of life during the three previous months and is specific for caregivers of children with asthma. It was developed in Spanish, and consists of 15 items that offer four response options each. Responses assign a higher score when the caregivers’ quality of life is worse. The instrument has been proven to have an adequate factorial structure to explore three quality of life dimensions: functional, emotional and socio-occupational. Four variables were obtained to be used in the analysis: total instrument score (IFABI-T), and the scores for each of the sub-scales: functional (IFABI-F), emotional (IFABI-E) and socio-occupational (IFABI-S). All scores ranged between 1 (better) and 4 (worse quality of life). Only fully completed questionnaires were included in the analyses.
- (2)
Caregiver's perception of asthma symptoms was evaluated using the Spanish-language version11,12 of the questionnaire on the perception of symptoms and incapacity in asthma (PSI) by Usherwood et al.13 This questionnaire explores the perception that parents/caregivers have of the symptoms and incapacity due to their child's asthma in the past three months. It consists of 17 items with five response options each and includes three dimensions: daytime symptoms, night-time symptoms and incapacity. The reliability and validity of the Spanish-language version has been verified in caregivers of children between 2 and 15 years old. The mean scores for the complete instrument (PSI-T) were obtained as well as for each of the subscales: daytime symptoms (PSI-D), night-time symptoms (PSI-N) and incapacity due to asthma (PSI-I), all on a scale between zero (lesser intensity of symptoms) and 4 (greater intensity).
- (3)
Family functioning was assessed using the “family APGAR” instrument by Smilkenstein,14 which has a validated version in Spanish.15 “APGAR” is the acronym for family functioning components that the instrument researches (adaptability, partnership, growth, affection and resolve). This self-administrated questionnaire has five items with three possible response options. The scores obtained vary between 1 (worse) and 3 (better family function). All results were categorised as good family functioning (score=3) or poor family functioning (score<3).
- (4)
Asthma Control was assessed by an experienced paediatrician, based on clinical criteria (Third National Asthma Expert Panel Report, NAEPP-3).3 To assess asthma control in children >6 years of age, pulmonary function was included, which was studied by means of spirometry in keeping with the recommendations of the American Thoracic Society and the European Respiratory Society,16 using the reference values proposed by the Global Lung Function Initiative.17 Asthma control was classified on three levels: good, partial and poorly controlled.
- (5)
Caregivers’ assessment of changes in their quality of life between two evaluations. The global rating of change index (GRC) was used, which is a procedure employed in other assessment studies9; it uses a 15-point scale of change, between −7 (severe worsening of quality of life) and +7 (huge improvement). When the caregivers indicated a change between −1 and +1, it was considered not to be any change. A score of −3, −2, +2 or +3 was considered a minimal significant change in the caregivers’ quality of life.
During a first study visit, the inclusion criteria were verified, socio-demographic data were collected and the caregiver's quality of life, perception of symptoms and incapacity, family functioning and control of asthma were assessed. During a second visit, 16 weeks later, a new assessment of the caregivers’ quality of life, perception of the symptoms and control of asthma was performed, and the global rating of change index was also determined.
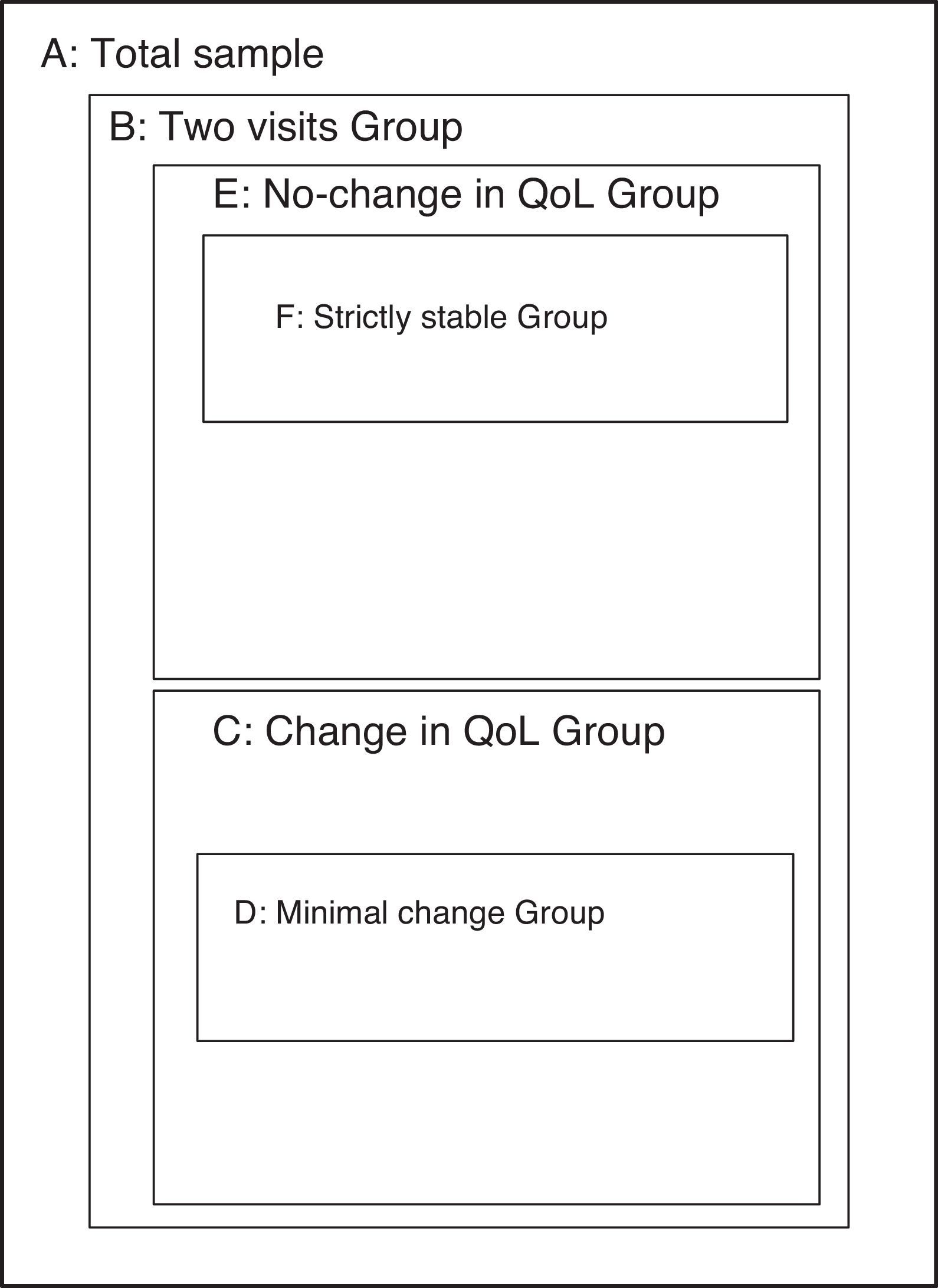
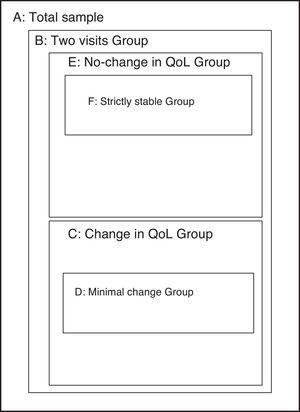
AnalysisThis study sought to include all important metric characteristics in an instrument intended to assess the quality of life, covering both the evaluative properties (which grant the capacity to detect important within-subject changes) such as discriminative properties (ability to detect between-subject differences). To study each of these properties, specific sub-groups from the sample were used (Fig. 1).
Sub-samples used in the various sections of the analysis. (A) Total sample (n=462). (B) Sub-sample with data from the second visit (n=401). (C) Sub-sample with change in quality of life between visits 1 and 2 (Global Rating of Change, GRC, outside the range −1 to +1) (n=269). (D) Sub-sample with minimal significant change in quality of life between visits 1 and 2 (GRC of −3, −2, +2 or +3) (n=49). (E) Sub-sample without change in the quality of life between visits 1 and 2 (GRC between −1 and +1) (n=148). (F) Sub-sample with strict definition of quality of life stability between visits 1 and 2 (see text) (n=47).
In the full sample (sample A), the internal consistency was analysed (Cronbach's alpha) as well as the cross-sectional construct validity. This latter was studied using the association of the IFABI-R scores with the PSI (Spearman correlation) and with the degree of asthma control and with family functioning (using variance analysis).
Sub-sample B (patients who came to a second visit, with the same caregiver providing information on both visits) was used to study the longitudinal construct validity, analysing the correlation (Spearman coefficient) of the changes observed in the IFABI-R scores with the corresponding changes in the PSI scores and changes in the degree of asthma control.
In sub-sample C (caregivers who had a GRC outside the range −1 to +1, that is, who referred to significant changes in their asthma-related quality of life), the sensitivity to change of IFABI-R was studied by analysing the differences in the initial and final scores using paired t-tests. Moreover, we studied whether the change in the IFABI-R scores (in absolute values) between the first and second visits differed among those caregivers with a change in their asthma-related quality of life and those without any significant change (sub-sample C vs. sub-sample E), analysing the differences using unpaired t-tests. Finally, the correlation between changes in IFABI-R with the corresponding score for changes in GRC was studied using Spearman correlation coefficient.
In sub-sample D (caregivers who experienced a minimal significant change in their quality of life, that is, who had a GRC of −3, −2, +2 or +3) a minimal important difference was identified in the IFABI-R score as the median of the change (in absolute values) taking place in that group. This is similar to the procedure applied in the validation of similar instruments.9
Finally, the test–retest stability of IFABI-R scores between the first and second visits were determined using single measure intra-class correlation coefficients based on a one-way random model.18 Given that the time between the two visits was too prolonged to accept complete stability in all of sub-sample E (see Fig. 1), the test–retest stability was studied in the sub-sample of caregivers who complied with a set of strict criteria (sub-sample F): absence of important changes in the asthma-related caregivers’ quality of life (GRC score between −1 and +1), variation in the PSI score (total and for each of its scales) <1 standard deviation of the initial score, lack of change in the degree of asthma control and lack of major family changes during that period (death, divorce, etc.).
The statistically analysis was performed using SPSS 15.0.
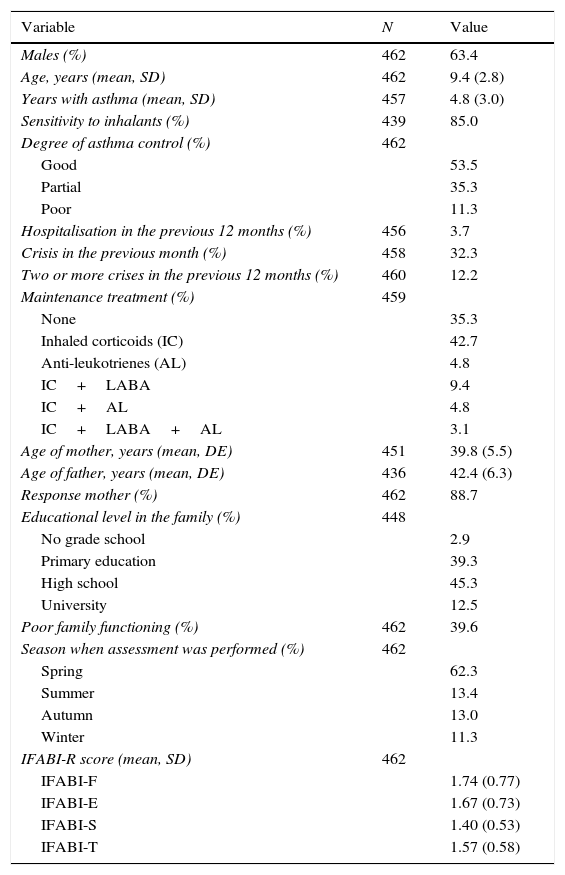
ResultsInitially, the main caregivers of 510 children were included; of these, 462 completed the IFABI-R correctly on the first visit. Those children not included had no significant differences with respect to sex, age, years with asthma, degree of asthma control, sensitivity to inhalants, season of evaluation, type of maintenance treatment, educational level in the family, family functioning or age of the parents. The most important demographic information is shown in Table 1.
Characteristics of the sample.
| Variable | N | Value |
|---|---|---|
| Males (%) | 462 | 63.4 |
| Age, years (mean, SD) | 462 | 9.4 (2.8) |
| Years with asthma (mean, SD) | 457 | 4.8 (3.0) |
| Sensitivity to inhalants (%) | 439 | 85.0 |
| Degree of asthma control (%) | 462 | |
| Good | 53.5 | |
| Partial | 35.3 | |
| Poor | 11.3 | |
| Hospitalisation in the previous 12 months (%) | 456 | 3.7 |
| Crisis in the previous month (%) | 458 | 32.3 |
| Two or more crises in the previous 12 months (%) | 460 | 12.2 |
| Maintenance treatment (%) | 459 | |
| None | 35.3 | |
| Inhaled corticoids (IC) | 42.7 | |
| Anti-leukotrienes (AL) | 4.8 | |
| IC+LABA | 9.4 | |
| IC+AL | 4.8 | |
| IC+LABA+AL | 3.1 | |
| Age of mother, years (mean, DE) | 451 | 39.8 (5.5) |
| Age of father, years (mean, DE) | 436 | 42.4 (6.3) |
| Response mother (%) | 462 | 88.7 |
| Educational level in the family (%) | 448 | |
| No grade school | 2.9 | |
| Primary education | 39.3 | |
| High school | 45.3 | |
| University | 12.5 | |
| Poor family functioning (%) | 462 | 39.6 |
| Season when assessment was performed (%) | 462 | |
| Spring | 62.3 | |
| Summer | 13.4 | |
| Autumn | 13.0 | |
| Winter | 11.3 | |
| IFABI-R score (mean, SD) | 462 | |
| IFABI-F | 1.74 (0.77) | |
| IFABI-E | 1.67 (0.73) | |
| IFABI-S | 1.40 (0.53) | |
| IFABI-T | 1.57 (0.58) |
SD, standard deviation; LABA, long-acting beta-adrenergic; IFABI-F, functional sub-scale for IFABI-R; IFABI-E, emotional sub-scale for IFABI-R; IFABI-S, socio-occupational sub-scale for IFABI-R; IFABI-T, total IFABI-R score.
Cronbach's alpha (95% confidence interval) for the whole instrument was 0.941(0.931–0.950), 0.905 (0.889–0.919) for the functional subscale, 0.883 (0.866–0.899) for the emotional subscale, and 0.883 (0.863–0.902) for the socio-occupational subscale.
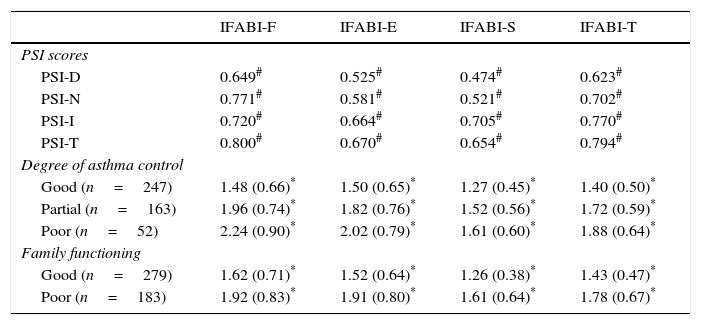
Cross-sectional construct validityTable 2 shows the correlation coefficients between IFABI-R and PSI scores and the association of the IFABI-R scores with the degree of asthma control and family adjustment.
IFABI-R discriminative capacity: cross-sectional construct validity.
| IFABI-F | IFABI-E | IFABI-S | IFABI-T | |
|---|---|---|---|---|
| PSI scores | ||||
| PSI-D | 0.649# | 0.525# | 0.474# | 0.623# |
| PSI-N | 0.771# | 0.581# | 0.521# | 0.702# |
| PSI-I | 0.720# | 0.664# | 0.705# | 0.770# |
| PSI-T | 0.800# | 0.670# | 0.654# | 0.794# |
| Degree of asthma control | ||||
| Good (n=247) | 1.48 (0.66)* | 1.50 (0.65)* | 1.27 (0.45)* | 1.40 (0.50)* |
| Partial (n=163) | 1.96 (0.74)* | 1.82 (0.76)* | 1.52 (0.56)* | 1.72 (0.59)* |
| Poor (n=52) | 2.24 (0.90)* | 2.02 (0.79)* | 1.61 (0.60)* | 1.88 (0.64)* |
| Family functioning | ||||
| Good (n=279) | 1.62 (0.71)* | 1.52 (0.64)* | 1.26 (0.38)* | 1.43 (0.47)* |
| Poor (n=183) | 1.92 (0.83)* | 1.91 (0.80)* | 1.61 (0.64)* | 1.78 (0.67)* |
IFABI-F, functional sub-scale for IFABI-R; IFABI-E, emotional sub-scale for IFABI-R; IFABI-S, socio-occupational sub-scale for IFABI-R; IFABI-T, total IFABI-R score; PSI, questionnaire about perception of symptoms and incapacity in asthma; PSI-D, PSI sub-scale for daytime symptoms; PSI-N, PSI sub-scale for night-time symptoms; PSI-I, PSI sub-scale for incapacity due to asthma; PSI-T, total PSI score.
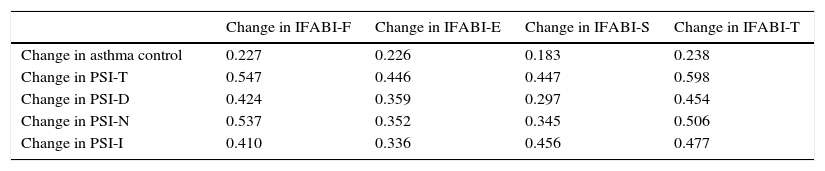
Sub-sample B included the caregivers of 401 children (64.1% males) with a mean age (standard deviation, SD) of 9.52 (2.83) years. The correlation coefficients of changes in the IFABI-R and changes in asthma control and PSI scores are shown in Table 3.
IFABI-R evaluative capacity: longitudinal construct validity.
| Change in IFABI-F | Change in IFABI-E | Change in IFABI-S | Change in IFABI-T | |
|---|---|---|---|---|
| Change in asthma control | 0.227 | 0.226 | 0.183 | 0.238 |
| Change in PSI-T | 0.547 | 0.446 | 0.447 | 0.598 |
| Change in PSI-D | 0.424 | 0.359 | 0.297 | 0.454 |
| Change in PSI-N | 0.537 | 0.352 | 0.345 | 0.506 |
| Change in PSI-I | 0.410 | 0.336 | 0.456 | 0.477 |
IFABI-F, functional sub-scale for IFABI-R; IFABI-E, emotional sub-scale for IFABI-R; IFABI-S, socio-occupational sub-scale for IFABI-R; IFABI-T, total IFABI-R score; PSI, questionnaire about perception of symptoms and incapacity in asthma; PSI-D, PSI sub-scale for daytime symptoms; PSI-N, PSI sub-scale for night-time symptoms; PSI-I, PSI sub-scale for incapacity due to asthma; PSI-T, total PSI score.
The statistics are Spearman rank correlation coefficient (ρ). All contrasts are significant (p<0.001).
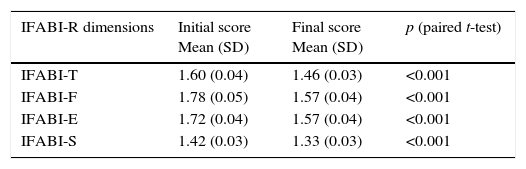
Sub-sample C included the caregivers of 269 children (62.8% males) with a mean age (SD) of 9.32 years (2.89). The differences between the initial and final IFABI-R scores were significant (p<0.001) and are shown in Table 4. Sub-sample E included 148 children (65.5% males) with a mean age (SD) of 9.74 (2.71) years. Changes (mean, SD) in the absolute value of IFABI-R scores between the two visits were compared for sub-samples C and E. These were: IFABI-T 0.30 (0.29) vs. 0.24 (0.29), p=0.050; IFABI-F 0.48 (0.51) vs. 0.41 (0.42), p=0.167; IFABI-E 0.37 (0.39) vs. 0.27 (0.35), p=0.011; IFABI-S 0.26 (0.32) vs. 0.21 (0.31), p=0.135, respectively. The correlation coefficients between changes in IFABI-R scores and GRC were: IFABI-T=−0.153 (p=0.002); IFABI-F=−0.154 (p=0.002); IFABI-E=−0.114 (p=0.023); IFABI-S=−0.065 (p=0.193).
IFABI-R evaluative capacity: sensitivity to change.
| IFABI-R dimensions | Initial score Mean (SD) | Final score Mean (SD) | p (paired t-test) |
|---|---|---|---|
| IFABI-T | 1.60 (0.04) | 1.46 (0.03) | <0.001 |
| IFABI-F | 1.78 (0.05) | 1.57 (0.04) | <0.001 |
| IFABI-E | 1.72 (0.04) | 1.57 (0.04) | <0.001 |
| IFABI-S | 1.42 (0.03) | 1.33 (0.03) | <0.001 |
IFABI-F, functional sub-scale for IFABI-R; IFABI-E, emotional sub-scale for IFABI-R; IFABI-S, socio-occupational sub-scale for IFABI-R; IFABI-T, total IFABI-R score; SD, standard deviation.
Sub-sample D included the caregivers of 49 children (61.2% males) with a mean age (SD) of 8.98 (2.84) years. The absolute value of the IFABI-R scores changes between the first and second visit (median) was 0.27 for IFABI-T, 0.33 for IFABI-F, 0.20 for IFABI-E and 0.20 for IFABI-S.
Test–retest stabilitySub-sample F included the caregivers of 47 children (66% males) with a mean age (SD) of 9.95 (2.52) years. The interclass correlation coefficient (95% confidence interval) was 0.929 for IFABI-T (0.877–0.960, p<0.001), 0.834 for IFABI-F (0.722–0.904, p<0.001), 0.928 for IFABI-E (0.874–0.959, p<0.001) and 0.893 for IFABI-S (0.817–0.939, p<0.001).
DiscussionThis study has confirmed certain properties of the IFABI-R that were described before in the initial study, and has expanded information regarding other metric qualities for aspects that had not been studied previously. These results prove the validity, reliability and responsiveness of the IFABI-R instrument, which seems adequate to assess the quality of life of caregivers of children with asthma.
An instrument with evaluative capacity must be able to show the changes that come about in each subject (sensitivity to change) and have adequate longitudinal construct validity, proving that the changes observed with the instrument are simultaneous with the changes found in other related constructs. IFABI-R meets all of these requirements. In-depth observation of the behaviour of each sub-scale for IFABI-R shows that the evaluative performance of the socio-occupational sub-scale is weaker than the other two sub-scales; obvious differences were observed between the initial and final measures when there was a change in the quality of life, but the changes observed in IFABI-S fail to have a good correlation with a global rate of change index and these were not clearly different among the subjects with stable quality of life and those whose quality of life changed.
An instrument with a good discriminative capacity must offer adequate reliability, proven by means of test–retest stability, and have good cross-sectional construct validity. All of these characteristics have been proven in this study, both for the global assessment for IFABI-R as well as for each of the sub-scales.
From the analysis performed, a change of more than 0.3 points in the global IFABI-R score and the sub-scales scores seems to be the best cut-off to identify a minimal important change in the quality of life of caregivers of children with asthma. Although other studies have chosen the mean of the change observed as the cut-off point to identify a minimal important change,9 we have used the median because it is a statistic that makes better sense in samples with non-normal distribution.
The main limitation of this work lies in the difficulty to guarantee stability in the caregivers’ quality of life when studied 16 weeks apart, and this makes it difficult to verify test–retest stability. To overcome this limitation, the analysis has been limited to the test–retest stability for a sub-sample that meets very strict stability criteria (sub-sample F).
In conclusion, the IFABI-R has evaluative and discriminative properties that make this instrument a useful instrument in the study of the quality of life of caregivers of children with asthma.
Conflict of interestThe authors have no conflict of interest to declare.
FundingHealthcare Research Project PI11/02122, Instituto de Salud Carlos III, Ministry of Education and Competitiveness, Government of Spain.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Healthcare Research Project PI11/02122, Instituto de Salud Carlos III, Ministry of Education and Competitiveness, Government of Spain.