The anti-inflammatory effect of high-dose inhaled corticosteroids (ICS) in children with asthma exacerbation is unknown. We aimed to investigate the efficacy of single-high dose ICS versus oral prednisone treatment followed by a course of six day high-dose ICS or oral prednisone (P) treatment on the concentrations of Cys-LTs and 8-isoprostane levels in the exhaled breath condensate (EBC) of children with asthma exacerbation.
MethodsNinety-four children with moderate–severe asthma exacerbation were evaluated with asthma scores, peak expiratory flow rate (PEF), forced expiratory volume in first second (FEV1) and exhaled Cys-LT and 8-isoprostane levels before and after treatment. EBC was collected from 52 patients before and four hours after treatment with inhaled fluticasone propionate (FP) (4000μg) or P and after six days of treatment with FP-1000μg/day or P. Cys-LTs and 8-isoprostane concentrations were determined using a specific immunoassay kit.
ResultsBoth single high-dose FP (n=59) and p (n=35) treatment resulted in a significant improvement in asthma score (p<0.0001), PEF (p<0.0001), and FEV1 (p<0.0001). Cys-LT concentration in the EBC decreased significantly both after the initial treatment (p=0.001), and at the end of the six-day period in the FP group (p<0.0001). 8-Isoprostane concentration was lower only after six days of treatment with FP-1000μg/day in the FP group (p=0.023). There was a significant decrease in exhaled Cys-LTs after four hours (p=0.012) and six days of P treatment (p=0.018) in children with asthma exacerbation.
ConclusionsHigh-dose ICS treatment may be useful in the treatment of children with asthma exacerbation. The effects start as early as after four hours. The suppression of Cys-LTs production contributes to the early effects. Suppression of both Cys-LTs and oxidants may favourably contribute to the effects observed later.
Acute exacerbations are common events for asthmatics and continue to be an important health problem. Although international guidelines recommend systemic corticosteroids besides repetitive administration of short acting inhaled bronchodilators in the management of asthma exacerbations, frequent administration may increase the risks associated with systemic corticosteroid treatment in some patients. Even though the use of inhaled steroids during an asthma exacerbation has been proposed as an alternative, there is some concern about its efficacy.1–3 While some authors have suggested that inhaled steroids seem to act faster than oral steroids on symptoms and airway obstruction,2,4–6 and the recent Cochrane review of early use of ICS in the emergency department treatment of acute asthma found that there was a reduction from 32 to 17 hospital admissions per 100 patients treated with ICS agents compared with placebo, the same Cochrane review concluded that there was not sufficient evidence to support using ICS agents alone as a replacement for systemic corticosteroid therapy in acute asthma attacks.7 In addition, its exact mechanism of action in this setting is largely unknown.
In a well designed study about the mechanism of high dose ICS, Belda et al. have shown that high-dose inhaled fluticasone appears to have a faster and stronger effect in reducing sputum eosinophils than oral prednisone and to be as effective as prednisone in reducing plasma exudation, bronchial obstruction, and symptoms in adults with asthma exacerbation.1
Inflammation and oxidative stress are essential parts of asthma pathophysiology. Cysteinyl leukotrienes (Cys-LTs) are potent constrictors and pro-inflammatory mediators.8,9 Higher levels of Cys-LTs have been found in bronchoalveolar lavage (BAL), induced sputum, and in exhaled breath condensate (EBC) of asthmatics, especially in patients with unstable asthma.8–12 In addition, it has been suggested that Cys-LTs play an important role in airway remodelling. Lex et al.13 reported that there is a significant relationship between EBC Cys-LTs and reticular basement membrane thickness in endobronchial biopsies in children with asthma. Moreover, it was shown that intravenous montelukast added to standard care in adults with asthma exacerbation produced a significant decrease in airway obstruction throughout two hours.14
Eosinophils, neutrophils, macrophages, and mast cells all produce reactive oxygen radicals causing an increased oxidative stress as a feature of airway inflammation in asthma.15 8-Isoprostane has been proposed as a good marker of oxidative stress due to its stability, specificity for lipid peroxidation and was shown to increase in asthma.15,16 Exhaled 8-isoprostane is elevated in asthmatic children, and this increase has not been shown to be affected by inhaled steroid therapy.12,17,18
EBC, as a non-invasive and safe technique, has great potential for monitoring airway inflammation and oxidative stress in asthmatic patients.19–22 In contrast to bronchial biopsy, BAL and induced sputum, EBC collection itself does not affect the airway and therefore can be repeated several times, thus allowing longitudinal follow-up of exhaled markers of airway inflammation as a measure for treatment response.21 It can be obtained with minimal risk, especially in patients with asthma exacerbation, where contra-indications exist for more invasive techniques. A significant correlation between 8-isoprostane and Cys-LT concentrations both in BAL and EBC have been observed.23,24
Exhaled Cys-LT and 8-isoprostane concentrations are increased during an asthma exacerbation25 and Baraldi et al. have shown that five days of oral prednisolone treatment reduced exhaled Cys-LT and 8-isoprostane concentration in children with an asthma exacerbation.25
We aimed to assess the effect of single-high dose ICS versus oral prednisone (P) treatment on both exhaled Cys-LT and 8-isoprostane levels and clinical response in children with moderate–severe asthma exacerbation.
Materials and methodsSubjectsThe subjects were children 6–18 years old who had a known history of asthma and who presented with an acute asthma exacerbation. Asthma exacerbation was defined as an increase in symptoms, such as cough, wheezing, shortness of breath or chest tightness, and β2-agonist use.26 In order to exclude children with lower respiratory tract infections, we did not include children with fever or fine rales with auscultation.
The asthmatic children were evaluated with childhood asthma control test (c-ACT) score and the severity at initial presentation and at follow-up was expressed as “asthma score”;27 peak expiratory flow rate (PEF) was evaluated with PEF-meter and forced expiratory volume in first second (FEV1) measured by a dry spirometer (Sensor Medics, Vmax22, CA, USA). The asthma exacerbation score used in our study is a modification of the asthma score by Qureshi F et al., published by the National Institutes of Health.28 The interrater reliability of the scoring system, tested in 98 children in the emergency department was good.27 The overall asthma score (range 5–15 points) was calculated by adding the scores for each of the following five variables: respiratory rate, oxygen saturation, auscultation, retractions, and dyspnea. Only children with moderate (asthma score of 8–11) or severe (asthma score of 12–15) exacerbation of asthma were enrolled in the study. While enrolling children into our study, we used both asthma exacerbation score and GINA asthma exacerbation classification.26,28 In order to evaluate our patients with a validated classification system we classified our patients according to GINA asthma exacerbation classification and in order to follow treatment response with a numeric score we used asthma exacerbation score.26,28 All of our patients were in the moderate or severe asthma exacerbation classes according to both GINA classification and asthma exacerbation score.
Children who used systemic corticosteroids within three weeks prior to the study; those receiving ICS at a dose of ≥1000μg/day budesonide or equivalent; those demonstrating signs of lower respiratory tract infection or systemic diseases other than asthma and those who had a history of intubation for asthma exacerbation were excluded from the study.
Study designThe study was performed as a prospective trial. The personnel performing the ELISA tests were unaware of the clinical status of the cases and the protocol of the study.
Ninety-four children (62 boys and 32 girls) that presented to Gaziantep University Paediatric Allergy and Asthma Unit with asthma exacerbation between January 2009 and April 2010 were included in our study. Fifty-nine children were included into the high-dose fluticasone propionate (FP) group and 35 children were included into the oral prednisone (P) group in the clinical part of the study. Exhaled breath condensates were obtained from 34 patients in the FP group and 18 children in the P group for pre-treatment analysis in the exhaled breath condensate study.
Study protocolNinety-four children with moderate or severe asthma exacerbation were included. Since Belda et al. showed the anti-inflammatory effect of 4000mcg FP by showing the improvement in the sputum eosinophil number in patients with acute asthma, we chose to treat our patients with 4000mcg FP.1 59 children in the FP group were treated with nebulised FP 2mg/ml solution at a single dose of 4000μg, and 35 children in the P group were treated with oral prednisone at a single dose of 1mg/kg. Both groups were treated with nebulised albuterol solution at a dose of 0.15mg/kg followed by two nebulisations of albuterol 20min apart. After the first hour of treatment, albuterol was given hourly until a decision was made to admit or discharge the patient. Inhaled medications were administered with the use of a jet nebuliser (Respirair F400, Flaemnuova, S. Martino, Italy). PEF, FEV1 and asthma scores were determined before administration of FP or P and albuterol and then hourly during the first four hours of treatment. Each evaluation was made before each albuterol administration. A decision to admit or discharge the child was made by the attending physician. Children were discharged if they demonstrated a PaO2 ≥94% at room air and had a FEV1 of ≥70%. After discharge, the children in the FP group were treated with inhaled FP at a dose of 1000μg/daily with pressurised metered dose inhaler and spacer; and P group was treated with oral P 1mg/kg/day for six days at home. During this period, patients discontinued their usual regular ICS treatment. PEF, asthma score, and pulmonary function tests were determined after six days of treatment with FP-1000μg/daily or oral P.
The EBC was collected before treatment, four hours after the initial treatment with inhaled FP-4000μg or oral P, and at the end of the six days of treatments.
The primary outcome of the study was the change in EBC Cys-LTs and 8-isoprostane level after four hours of treatment with single-high dose FP-4000μg or oral P. Secondary outcomes were the effects of treatment on pulmonary functions and asthma scores.
Study measurements and proceduresCollection of EBCA commercially available device, EcoScreen (Jaeger, Wurzburg, Germany), was used for the collection of EBC. In this collection system, exhaled air enters and leaves the chamber through a two-way, non-breathing valve, and saliva is filtered. Expiratory air flows through a lamellar condenser that is surrounded by a cooling cuff. The interior temperature is −15°C or cooler. EBC was collected according to the ATS/ERS Task Force recommendations.29 All subjects breathed in a relaxed manner (tidal breathing) for 15min. Approximately 1ml of breath condensate was collected in sterile Eppendorf tubes and immediately upon collection the samples were frozen on dry ice and stored in −80°C until measurement within six months of collection.
Measurements of EBCExhaled 8-isoprostane concentrations were quantified using a specific enzyme immunoassay kit (Cayman Chemicals, Ann Arbour, MI, USA) as previously described. The detection limit was 2.7pg/ml.
Cys-LT concentrations were determined using a specific immunoassay kit (Cayman Chemicals, Ann Arbour, MI, USA), which measures three leukotrienes, LTC4, D4, E4 in a single measurement as previously described.11 The detection limit was 13pg/ml.
EthicsAll study procedures were done in accordance with a protocol previously approved by the Ethics Committee of Gaziantep University. All parents provided written informed consent for the study procedures and the children gave consent.
Statistical analysesA statistical software package was used for all data analysis and comparisons (SPSS v 11.5). The data were tested for assumptions of normalcy, and differences between the groups were compared with Mann–Whitney U test and Kruskal–Wallis accompanied by Dunn's multiple comparison. Spearman's rank correlation coefficient was applied to investigate the correlation between different parameters. Multiple linear regressions were applied whenever needed to adjust for age, gender as potential confounders. A p value of less than 0.05 was considered significant.
Sample size was estimated using a power calculation based on 50% reduction in Cys-LT and 8-isoprostane measures which showed that at least 12 patients would be required to detect a significant difference between baseline levels and fourth hour levels at 80% power level and an alpha error of 5%.
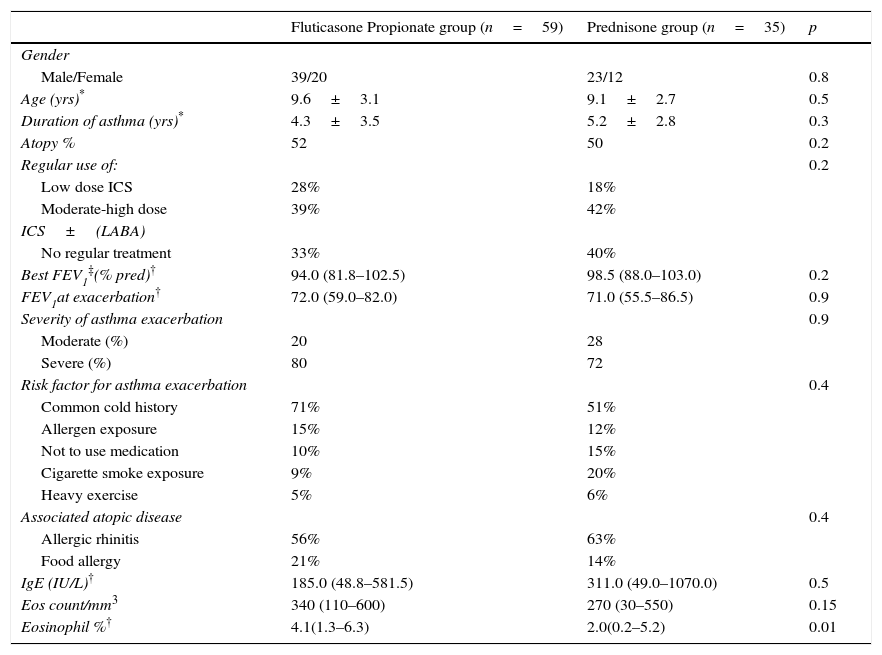
ResultsFifty-nine and 35 children were included in the FP and P groups, respectively, in the clinical part of our study. The main characteristics of the study population are given in Table 1. During the six day period of treatment, none of the children was hospitalised.
Main characteristics of the study population at the time of asthma exacerbation.
| Fluticasone Propionate group (n=59) | Prednisone group (n=35) | p | |
|---|---|---|---|
| Gender | |||
| Male/Female | 39/20 | 23/12 | 0.8 |
| Age (yrs)* | 9.6±3.1 | 9.1±2.7 | 0.5 |
| Duration of asthma (yrs)* | 4.3±3.5 | 5.2±2.8 | 0.3 |
| Atopy % | 52 | 50 | 0.2 |
| Regular use of: | 0.2 | ||
| Low dose ICS | 28% | 18% | |
| Moderate-high dose | 39% | 42% | |
| ICS±(LABA) | |||
| No regular treatment | 33% | 40% | |
| Best FEV1‡(% pred)† | 94.0 (81.8–102.5) | 98.5 (88.0–103.0) | 0.2 |
| FEV1at exacerbation† | 72.0 (59.0–82.0) | 71.0 (55.5–86.5) | 0.9 |
| Severity of asthma exacerbation | 0.9 | ||
| Moderate (%) | 20 | 28 | |
| Severe (%) | 80 | 72 | |
| Risk factor for asthma exacerbation | 0.4 | ||
| Common cold history | 71% | 51% | |
| Allergen exposure | 15% | 12% | |
| Not to use medication | 10% | 15% | |
| Cigarette smoke exposure | 9% | 20% | |
| Heavy exercise | 5% | 6% | |
| Associated atopic disease | 0.4 | ||
| Allergic rhinitis | 56% | 63% | |
| Food allergy | 21% | 14% | |
| IgE (IU/L)† | 185.0 (48.8–581.5) | 311.0 (49.0–1070.0) | 0.5 |
| Eos count/mm3 | 340 (110–600) | 270 (30–550) | 0.15 |
| Eosinophil %† | 4.1(1.3–6.3) | 2.0(0.2–5.2) | 0.01 |
ICS=inhaled corticosteroids, LABA=long acting β2 agonists, * Mean±Standard deviation.
†: Median (interquartile range), ‡: Highest value measured in the previous year.
Exhaled Cys-LT concentrations were obtained from 34, 32, and 23 patients in the FP group, and from 18, 16, and 16 patients in the P group for pre-treatment, fourth hour, and sixth day analysis, respectively. Baseline Cys-LTs were able to be detected in 30/34 children in the FP group and in 8/18 children in the P group. There was no significant difference between baseline Cys-LT levels of FP (n=30) and P (n=8) groups [33.6pg/ml (19.5, 74.7) vs. 83.4pg/ml (17.3, 274.4), (median, interquartile range), p=0.5].
There was no significant difference in age, sex, baseline FEV1%, FEV1 improvement, and baseline Cys-LT and 8-isoprostane levels between children with and without sixth day mediator analysis.
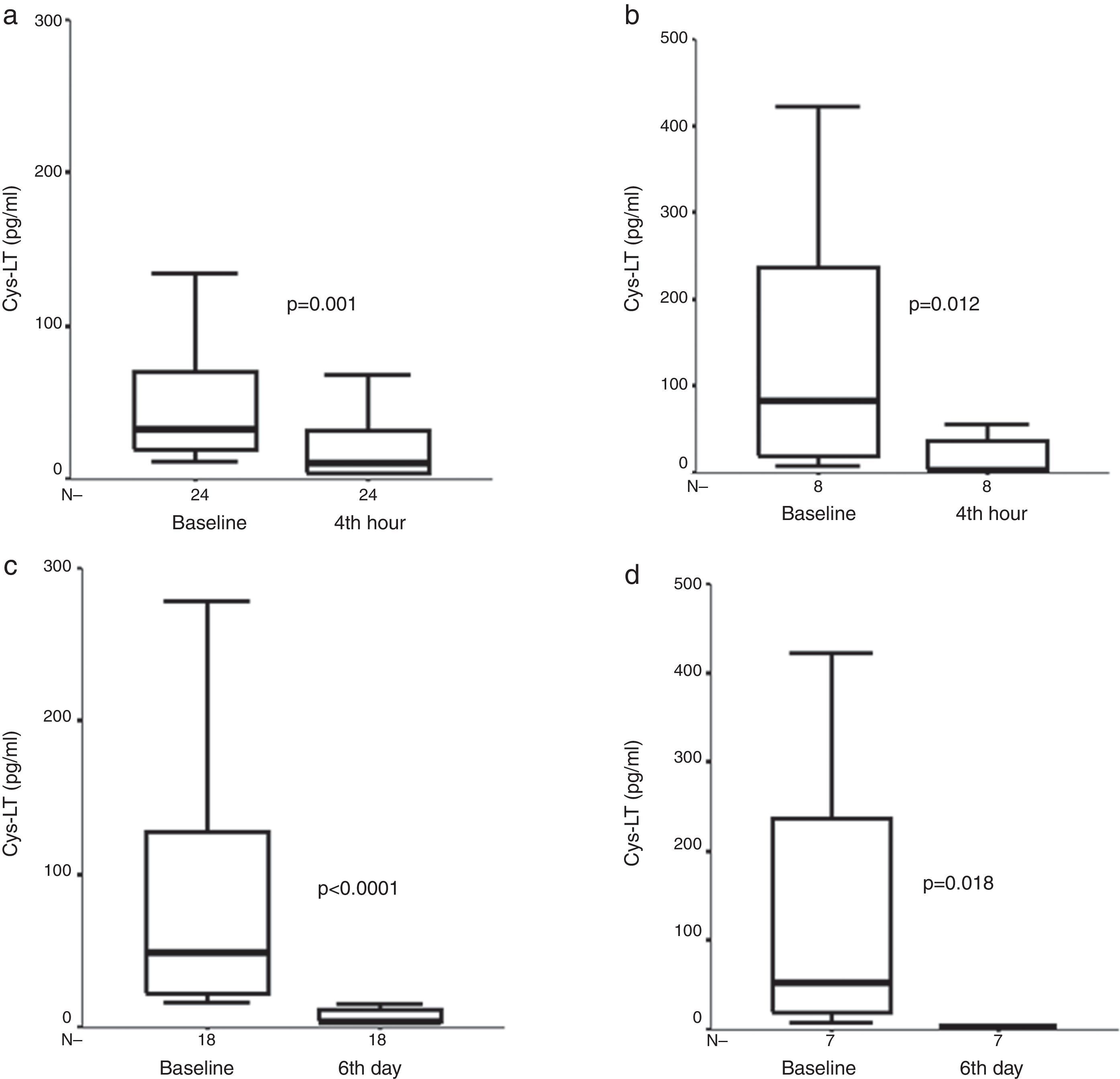
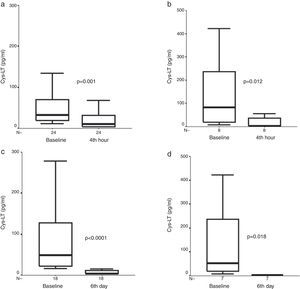
At four hours, there was a significant decrease in exhaled Cys-LT concentration [33.6pg/ml (19.5, 74.7) vs. 12.2 (3.9, 32.6), (median, interquartile range), (p=0.001, n=24)] in the FP group. A further decrease was observed on day six [3.9pg/ml (3.9, 15.7) vs. initial values, (p<0.0001, n=18)] in the FP group (Fig. 1).
Exhaled Cys-LT measures before, (a) after four hours of treatment with inhaled fluticasone propionate (FP), and (b) after four hours of treatment with oral prednisone (P), and exhaled Cys-LT measures before, (c) after six days of treatment with inhaled FP, and (d) after six days of treatment with oral P in children with asthma exacerbation. Median and interquartile range values are shown.
There was a significant decrease in exhaled Cys-LT concentrations at four hours and sixth day [83.4pg/ml (17.3, 274.4) vs. 3.9 (3.9, 46.5) vs. 3.9 (3.9, 3.9), (baseline vs. fourth hour vs. sixth day, respectively), (p=0.012, n=8), and (p=0.018, n=7), for fourth hour and sixth day analysis, respectively] in the P group (Fig. 1).
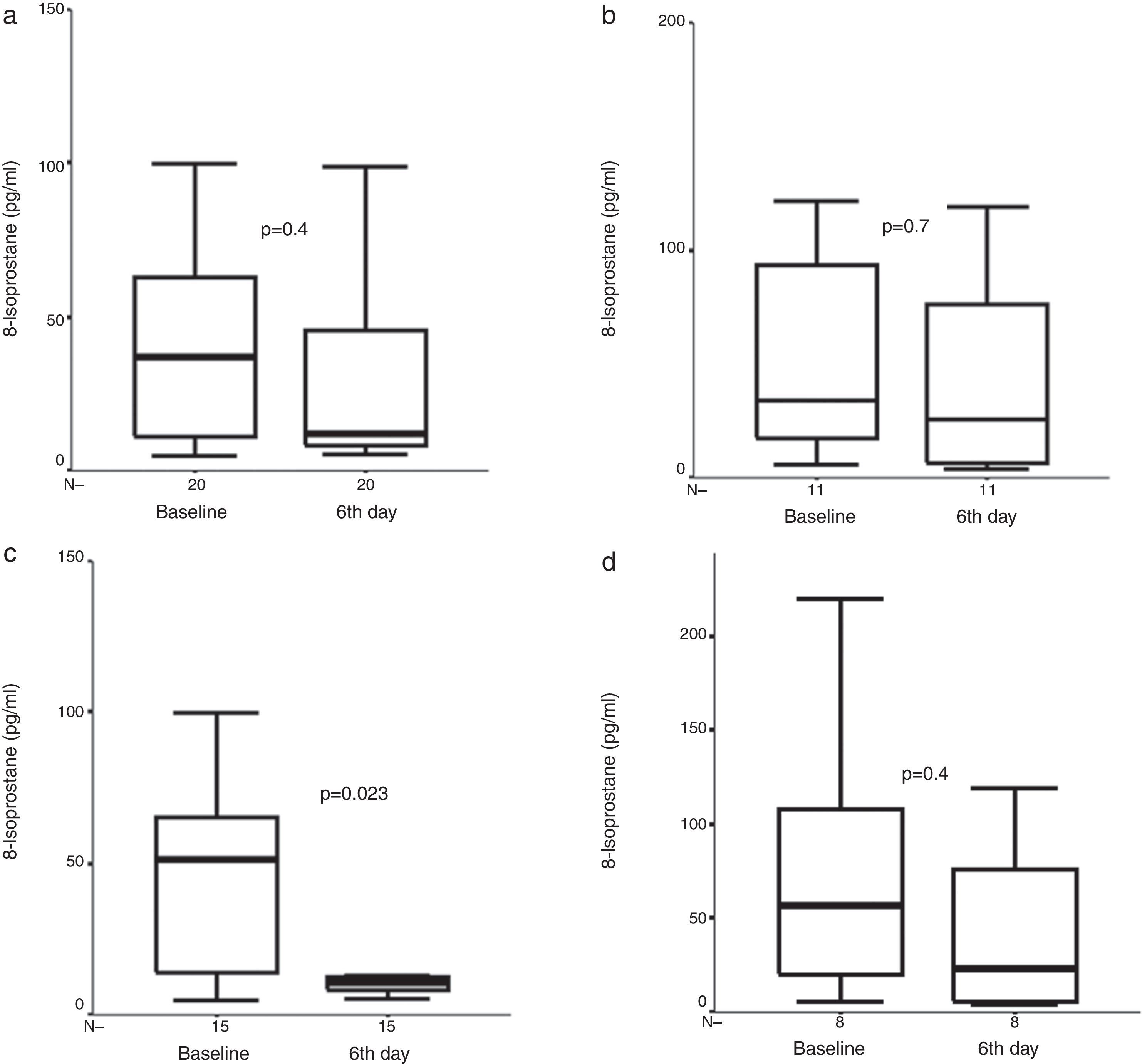
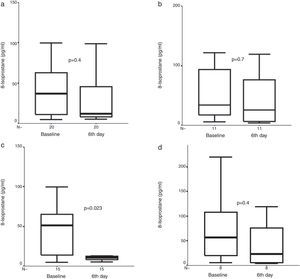
Exhaled 8-isoprostane concentrations were obtained from 34, 30, and 23 patients for pre-treatment, fourth hour, and sixth day analysis in the FP group, respectively. We could detect exhaled 8-isoprostane concentrations in all of the exhaled breath condensate samples. In contrast to Cys-LT, no significant difference was observed in the 8-isoprostane concentration after four hours or six days of FP treatment (Fig. 2). However, when moderate exacerbations were excluded and the analysis restricted to severe exacerbations only, a significant decrease in 8-isoprostane concentrations was observed after six days compared to baseline levels [10.4pg/ml (7.9, 17.4) vs. 44.0pg/ml (12.5, 61.8), p=0.023, n=15] in the FP group (Fig. 2).
Exhaled 8-isoprostane measures before and (a) after six days of treatment with inhaled fluticasone propionate (FP) in all study group, and (b) after six days of treatment with oral prednisone (P) in all study group, and exhaled 8-isoprostane measures before, and (c) after six days of treatment with inhaled FP in children with severe asthma exacerbation, and (d) after six days of treatment with oral prednisone (P) in children with severe asthma exacerbation. Median and interquartile range values are shown.
Exhaled 8-isoprostane concentrations were obtained from 17, 15, and 12 patients for pre-treatment, fourth hour, and sixth day analysis in the P group, respectively. We could detect exhaled 8-isoprostane concentrations in all of the exhaled breath condensate samples. There was no significant decrease in exhaled 8-isoprostane concentrations neither at four hours nor at sixth day [26.1pg/ml (14.4, 93.3) vs. 52.6pg/ml (14.7, 146.6) vs. 22.9 (5.0, 96.9), (baseline vs. fourth hour vs. sixth day, respectively), (p>0.05, n=14), (p>0.05, n=11)] in the P group (Fig. 2). There was also no significant decrease in exhaled 8-isoprostane concentrations neither at four hours nor at sixth day in the P group with severe asthma exacerbation (Fig. 2).
Baseline Cys-LTs and eosinophil percentages during asthma exacerbationBaseline Cys-LTs were able to be detected in 30/34 children in the FP group and in 8/18 children in the P group. There was a significant difference in the eosinophil percentages between FP and P groups (Table 1). Lower eosinophil levels may be related with lower detectability of exhaled Cys-LTs in the prednisone group. When we analysed eosinophil levels in children with detectable Cys-LTs, there was no significant difference between two treatment groups.
Correlation analysesThe percentage reduction in Cys-LTs correlated significantly with baseline Cys-LT levels at four hours (r=0.58, p=0.001), and six days (r=0.73, p=0.0002) in the FP group. Similarly, the percentage reduction in 8-isoprostane at six days correlated significantly with baseline 8-isoprostane levels in the FP group (r=0.83, p<0.0001).
There was no significant correlation between FEV1, PEF levels and exhaled Cys-LT measures. There was no significant correlation between improvement in asthma score, PEF levels, and reduction in exhaled 8-isoprostane and Cys-LT measures.
There was no significant difference in the eosinophil counts between FP and P groups (Table 1). But, even though there was no significant difference in the frequency of atopy and IgE levels between FP and P groups, there was a significant difference in the eosinophil percentages (Table 1). Although there was a significant difference in the eosinophils% between the P and FP groups, we could not demonstrate any significant correlation between eosinophil levels and clinical improvement in pulmonary function test measure, asthma exacerbation scores or change in exhaled mediators.
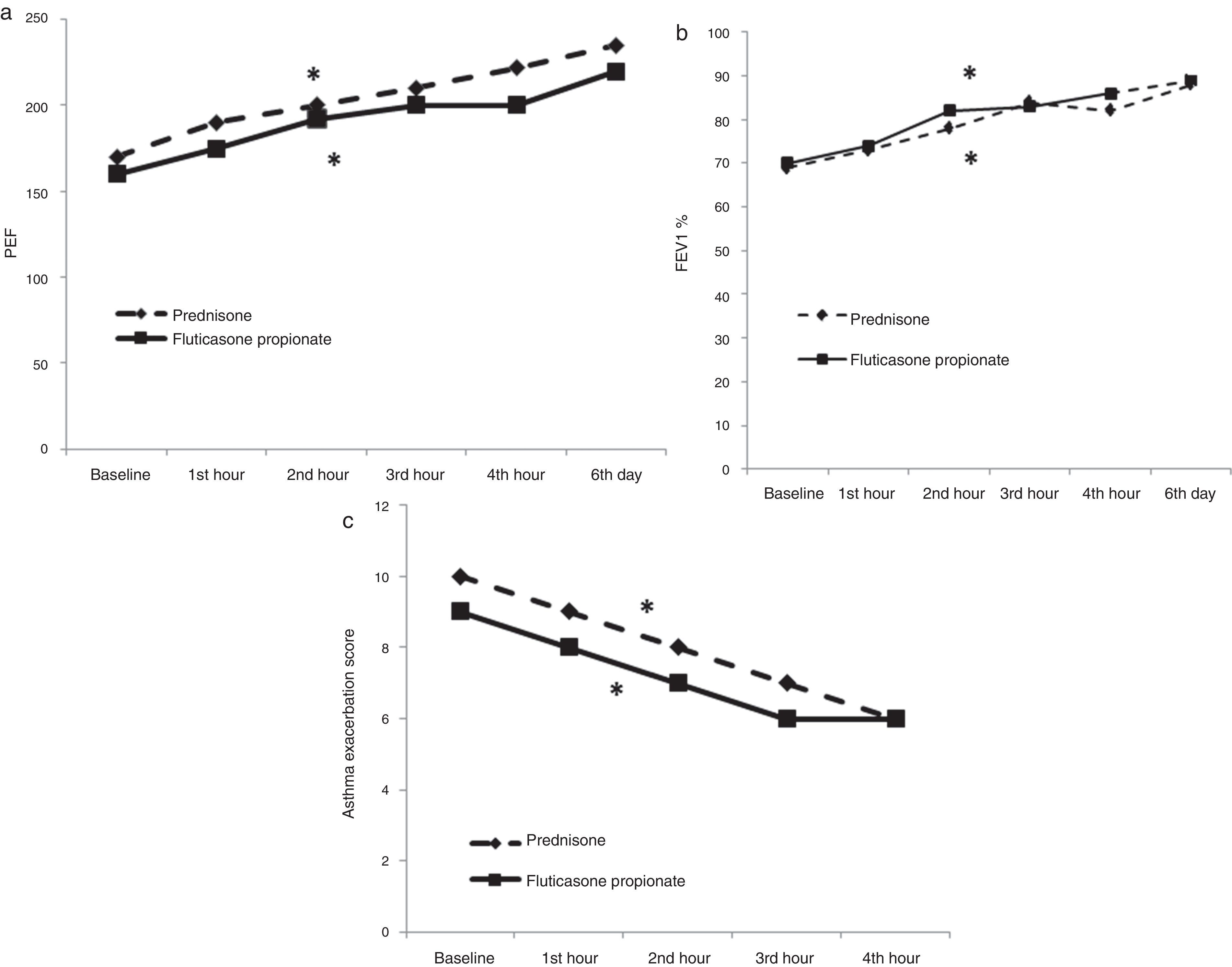
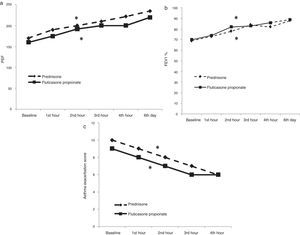
Effect of treatment on asthma score and airflow obstructionFP-4000μg treatment induced a significant improvement in asthma scores after four hours of treatment [from 9 (8, 10) to 6 (5, 7), p<0.0001] (Fig. 3). Improvement in symptom scores was accompanied by improvements in pulmonary function tests: PEF improved from [160L/min (115, 222) to 195 (135, 267), p<0.0001] at four hours and to 220 (160, 275) on day six (p<0.0001); FEV1 improved from [69% (56, 78) to 82% (75, 98), p<0.0001], at four hours and to 89% (81, 99), on day six (p<0.0001) (Fig. 3).
Oral P treatment induced a significant improvement in asthma scores after four hours of treatment [from 10 (9, 10) to 6 (5, 8), p<0.0001] (Fig. 3). Improvement in symptom scores was accompanied by improvements in pulmonary function tests: PEF improved from [170L/min (110, 215) to 222 (160, 253), p<0.0001] at four hours and to 200 (160, 265) on day six (p<0.0001); FEV1 improved from [69% (56, 85) to 82% (70, 94), p<0.0001], at four hours and to 93% (80, 103), on day six (p<0.0001) (Fig. 3).
Childhood asthma control test (c-ACT) scoresThere was a significant improvement in c-ACT scores [16.5(13, 20) vs. 21 (19, 23), (p<0.0001)] obtained one-month after asthma exacerbation treatment, when our patients were stable, compared to c-ACT scores on the day with asthma exacerbation.
Atopy and changes in the Cys-LT and 8-isoprostane concentrations and clinical outcomeThere was no significant difference in the changes in Cys-LTs and 8-isoprostane levels and clinical outcome between atopics and non-atopics in both treatment groups. (Data not shown).
No adverse events including fever, vomiting, increased dyspnoea, decreasing oxygen saturation, intubation or hospital admission was observed during treatment.
DiscussionOur study shows that both single-high dose inhaled FP-4000μg and oral P treatment improved asthma exacerbation score and pulmonary functions and that exhaled Cys-LT concentrations were reduced significantly as early as four hours after single-high dose FP in children with moderate-severe exacerbation. Cys-LT concentrations were further decreased after six days of FP-1000μg/day. Moreover, a significant decrease in Cys-LT concentrations was observed after four hours, and six days of P treatment. On the other hand, 8-isoprostane levels decreased only after six days of FP and only in those children with a severe asthma exacerbation. The percentage reduction in Cys-LTs and 8-isoprostane correlated significantly with baseline mediator levels.
It has been shown that Cys-LT levels increase above their baseline levels during asthma exacerbation9,30 and corticosteroids can selectively affect the increased leukotriene production31 even though they have no effect on BAL fluid levels in stable asthmatics.32,33 Regarding this issue, Shindo et al.31 found that 5-hydroperoxyeicosatetraenoic acid (5-HPETE) levels, which can be considered a measure of 5-lipoxygenase (5-LOX) activity, were significantly higher in the cytosol of blood eosinophils obtained from asthmatic patients during acute wheezing than in those in remission, and they were inhibited by prednisolone in a dose dependent fashion. In agreement with this in vitro study, we have shown a significant decrease in Cys-LT concentrations after four hours, and six days of P treatment in children with moderate-severe exacerbation. Moreover, we have shown that exhaled Cys-LT concentrations were reduced significantly four hours after single-high dose FP and an even more significantly after six days of FP-1000μg/daily in children with moderate–severe exacerbation.
Djukanovic et al. showed nicely that epithelial and mucosal mast cells and eosinophils and submucosal T lymphocytes were reduced significantly in association with clinical improvement following two weeks of therapy with 2000μg/day and followed by four weeks of therapy with 1000μg/day inhaled beclomethasone dipropionate.34 But the data concerning the acute effects of systemic corticosteroids and specifically of ICS on the airway inflammation are scarce. This may be partly due to the difficulty in obtaining bronchial secretions in dyspnoeic asthmatics1 during a naturally occurring asthma exacerbation. In one of the few studies, exhaled Cys-LTs and 8-isoprostane concentrations were shown to decrease after five days of oral prednisone treatment in children with asthma exacerbation.25 Our study supports this observation and shows that the same effect can be obtained with high-dose inhaled corticosteroid treatment as well as P treatment. Furthermore, ICS may have higher local anti-inflammatory effect compared to oral P as shown before.1,35 In the light of our finding, we may recommend not to stop usual regular ICS treatment while treating children with asthma exacerbation with systemic corticosteroids as suggested by Rowe et al.3 Furthermore, the early addition of ICS to the systemic corticosteroids in the emergency department and continuation of these preventive agents following discharge should be considered.3
The mechanism of the acute effect of ICS in an asthma exacerbation is not yet clear. One study reported a reduction in sputum eosinophils with 4000μg inhaled FP in two hours in adults with moderate asthma exacerbation.1 Gibson et al.35 showed that sputum eosinophils were reduced six hours after 2400μg inhaled budesonide treatment in adults with stable asthma. Aggarwal et al.36 showed that there is a correlation between sputum eosinophils and sputum Cys-LTs in adults with asthma. Cys-LTs are generated mainly by mast cells and eosinophils and they are capable of recruiting eosinophils.37 The lower concentration of Cys-LTs that we have observed can be due to two different factors. ICS may have decreased the eosinophil infiltration resulting in decreased production of Cys-LTs; or ICS may have decreased Cys-LTs by another mechanism and thus lead to decreased eosinophil recruitment. Even though these two mechanisms are not mutually exclusive we do not have eosinophil data to support these hypotheses.
Although it has been shown that sputum eosinophil count may predict inhaled corticosteroid responsiveness,38 Sont et al. have shown that there was no correlation between the number of infiltrating leucocytes such as mast cells, activated eosinophils, CD8+, and CD45RO+ cells in bronchial biopsy specimens from asthmatics on regular treatment with inhaled steroids and symptoms or lung function.39 Similarly, although there was a significant difference in the percentage of eosinophils between the P and FP groups, we could not demonstrate any significant correlation between eosinophil levels and clinical improvement in pulmonary function test measure, asthma exacerbation scores or change in exhaled mediators, and our data failed to show any correlation between the decrease in the Cys-LTs and clinical improvement. Therefore, there is reason to believe that other factors are also involved. Multiple factors may play a role in the clinical efficacy of high dose ICS. ICS can have two different effects on acute asthma patients: (1) the classic anti-inflammatory or genomic action, involving the modification of gene expression, occurring within hours or days; and (2) the non-genomic action, with a rapid onset in minutes, but of shorter duration.40 Kumar et al. showed that inhaled FP induced vasoconstriction in the airway mucosa within 30min and was of short duration (90min).41 There is strong evidence that classic receptors belonging to the nuclear receptor superfamily mediate non-genomic steroid effects in some cases.42 In the light of this information, lack of correlation between the decrease in the Cys-LTs and the clinical improvement does not indicate that Cys-LTs do not have any role in the therapeutic benefit of ICS in acute asthma. However, it must suggest that many other genomic and non-genomic factors may be interrelated with the clinical efficacy of ICS.
Although Cys-LTs might have a role in bronchoconstriction, the lack of a correlation between the Cys-LTs and pulmonary function measures suggests that other mediators or mechanisms might contribute to bronchoconstriction. In addition, CysLT receptor nature and the sensitivity of the airway smooth muscle may be involved in the determination of bronchoconstriction.
We were unable to show the efficacy of single high-dose inhaled FP or oral P in exhaled 8-isoprostane levels. Significant reduction of exhaled 8-isoprostane measures was shown only after six days of FP and only in children with severe asthma exacerbation. Our result seems to confirm the partial effect of ICS on 8-isoprostane levels, previously emphasised by other studies.17,18,43 Mondino et al. have shown that four weeks-duration ICS did not reduce exhaled 8-isoprostane in steroid-naive asthmatic children.18 Since ICS-naive children and children with ICS therapy have similar exhaled 8-isoprostane levels, it has been suggested that exhaled 8-isoprostane does not seem to be normalised by ICS.17,43 In support of this notion, Baraldi et al. have suggested that corticosteroids may not be fully effective in reducing oxidative stress in children with asthma exacerbation.25 Furthermore, we showed that single high-dose ICS treatment may be insufficient to control oxidative stress adequately in children with moderate-severe asthma exacerbation. Since uncontrolled oxidative stress may damage lungs and increase inflammation, the duration of the treatment may have to be extended to as long as six days to control the ongoing oxidant stress.
We observed a higher inhibition in patients with higher baseline mediator levels as was shown for 8-isoprostane levels before.25 This means that whenever baseline mediator levels increase, the reduction in this mediator levels may be much more prominent after anti-inflammatory treatment.25
It has been shown that the use of inhaled fluticasone in doses of up to 1000μg/day as long-term (at least 12 months) treatment of children with severe asthma did not substantially affect their adrenal function.44 But there is no data on the systemic adsorption of single dose inhaled-FP 4000mcg.
A limitation of our study is that the clinical and anti-inflammatory effects of different doses of ICS were not studied. Further placebo-controlled studies investigating the clinical and anti-inflammatory response to different doses of ICS treatment will answer if a flat dose response curve with high-dose ICS treatment is present in asthma exacerbation. Our study provides suitable pilot data for such studies. As supporting this topic, the Cochrane review of early use of ICS in the emergency department treatment of acute asthma found that inhaled corticosteroids helped to relieve asthma attacks, but, the most effective drug and dosage are unclear.7
Another limitation of our study is the lack of placebo group due to obvious ethical concerns. In this regard, day-to-day repeatability of exhaled eicosanoids was demonstrated in the previous studies.18,25 In the present study, we tried to decrease variability in exhaled mediators by collecting the EBC by the same staff, collecting the EBC at standard volume and duration, using same analytic technique, evaluating the same patient for monitoring treatment response. On the other hand, we evaluated oral P group as a standard guidelines-recommended treatment group to compare with single high-dose ICS treatment in children with asthma exacerbation. Furthermore, we demonstrated that monitoring treatment response was possible by EBC even in dyspnoeic children with exacerbation without increasing dyspnoea. Another limitation of our study is that, even though an early effect of ICS and mediators is shown, it fails to demonstrate the exact mechanism of action mainly because of the lack of supplemental cellular and molecular data, which is mainly due to the difficulties associated with obtaining tissue and sputum in this age group under circumstances necessitating emergency treatment.
In conclusion, our study shows that high-dose inhaled corticosteroid treatment is as effective as oral P treatment for children with moderate to severe asthma exacerbation. The effects start as early as four hours. Even though the suppression of Cys-LTs production contributes to the early effects and suppression of both Cys-LTs and oxidants may favourably contribute to the effects observed later, the exact mode of action and the underlying cellular and molecular mechanisms remain to be determined.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
FundingThis study was funded by a grant from Gaziantep University (no. TF.09.18).
Conflict of interestNone of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
The authors thank Emine Yılmaz, Sevil Kanat, Sevda Korkut, and Hasan Gokmen for their technical assistance.