Mast cell and basophiles are thought to be central to inflammation that has an allergic basis as allergens activate these cells in an IgE-dependent manner to generate mediators such as histamine, eicosanoids and cytokines. Phosphodiesterase (PDE) is known to exist as multiple molecular forms of enzyme that metabolise the second messengers. Studies of our own have shown that, of a variety of isoform-selective drugs, the PDE4-selective inhibitors, such as rolipram, attenuate the IgE-mediated release of histamine from human basophiles but not from human lung mast cells (HLMC).
The main aim of the present study was to characterise the type and role of PDEs regulating human skin mast cells by using selective and non-selective PDE inhibitors.
MethodsCells were pre-treated for 15min with these agents and then challenged with an optimal releasing concentration of anti IgE (1:300) for a further 25min for the release of histamine.
ResultsThe data show that all the selective PDE-inhibitor compounds (10−5M) were ineffective whereas the non-selective PDE inhibitor, theophylline (10−3M), inhibited histamine release from HSMC (74±4% inhibition; p<0.05). None of the selective PDE inhibitors had any effect on histamine release from HLMC whereas, in basophiles, compounds with activity at PDE 4 (rolipram, denbufylline, Ro-2017, Org 30029) were effective inhibitors of histamine release.
ConclusionThe data suggest that unlike most inflammatory cells, PDE-selective inhibitors are ineffective stabilisers of HSMC activity which is similar to HLMC.
At least eleven different classes of phosphodiesterase (PDE) have been identified based on structural and functional criteria.1–4 Enzymes within this family are found in most pro-inflammatory and immune cells where they are important regulators of the metabolism of cyclic nucleotides. Of the eleven PDE isoenzymes, types 3 and 4 hydrolyse cAMP and type 5, cGMP. The activity of PDEs can be modulated by inhibitors. These inhibitors can be divided into distinct categories: (a) classical non-specific inhibitors of PDE activity such as theophylline, and 3-isobutyl-1-methylxanthine (IBMX), and (b) selective PDE inhibitors such as 8-methoxy-methyl-IBMX (8-Me-IBMX; PDE 1 inhibitor), siguazodan (PDE 3 inhibitor), rolipram and denbufylline (PDE 4 inhibitors) and zaprinast (PDE 5 inhibitor). Pharmacological investigations, using selective and non-selective inhibitors of PDE isoenzymes, have shown that PDE type 3 and 4 inhibitors are more effective than PDE type 1, 2 and 5 inhibitors in inflammatory cells (8–9).
Since PDEs hydrolyse cAMP and cGMP, the mechanism by which PDE inhibitors act is to elevate levels of these cyclic nucleotides. Some PDEs hydrolyse cAMP (PDE 4) preferentially whereas others are cGMP-selective (PDE 5). Cyclic AMP and cyclic GMP, as intracellular messengers, play vital roles regulating inflammatory cell activity (9). Since cAMP and cGMP are almost unable to penetrate intact cell membranes, several hundred cyclic nucleotide analogues, with hydrophobic substituent, have been synthesised and have been widely used to elucidate the functional role of cAMP and cGMP signal cascades in biological systems.5,6 Four of these, namely, 2′-O-dibutyrylguanosine 3′,5′-cyclic monophosphate (Bu2-cGMP), 2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate (Bu2-cAMP), 8-bromo-cyclic 3′,5′-adenosine monophosphate (8-Br-cAMP) and 8-bromo-cyclic 3′,5′-guanosine monophosphate (8-Br-cGMP) have received major attention and are widely used as tools for testing the role of cAMP/cGMP and protein kinase A (PKA)/protein kinase G (PKG) in biological systems because cAMP and cGMP exert their physiological functions almost solely via activation of PKA and PKG, respectively.
The main aim of the present study was to characterise the type and role of PDEs regulating human skin mast cells by using selective and non-selective PDE inhibitors. Secondly, a comparison of the effects of these inhibitors on related cell types, human lung mast cells and basophiles, was also undertaken. Thirdly, an investigation of the effects of non-hydrolysable analogues of cAMP and cGMP on skin mast cells was also undertaken to determine the role of cyclic nucleotides in the regulation of skin mast cells.
Materials and methodsBuffersPhosphate-buffered saline (PBS) was employed in these studies. PBS contained (mM): NaCl 137; Na2HPO4·12H2O 8; KCl 2.7; KH2PO4 1.5. PBS–bovine serum albumin (BSA) was PBS which additionally contained: CaCl2·2H2O 1mM; MgCl2·6H2O 1mM; glucose 5.6mM; BSA 1mgml−1; DNase 15¿gml−1. PBS–human serum albumin (HSA) was PBS additionally supplemented with: CaCl2·2H2O 1mM; MgCl2·6H2O 1mM; glucose 5.6mM; HSA 30¿gml−1. The pH of all PBS buffers was titrated to 7.3.
Preparation of inhibitors and stimuliPhosphodiesterase (PDE) inhibitors were made up as follows: rolipram, IBMX, Org 30029, 8-methoxy-methyl-IBMX, denbufylline and Ro-201724 were made up as 10−1M and 10−2M stock solutions in 10% DMSO. Siguazodan and theophylline were made up as 1mM and 10mM stock solution, respectively, in +PBS. Zaprinast was prepared as a 100mM stock solution in 0.1M NaOH. Cyclic nucleotide analogues were made up as follows: 8-bromo-c-AMP, 8-bromo-c-GMP, Bu2-cAMP and Bu2-cGMP were prepared as 100mM stock solutions made in +PBS daily. The stimulus used in mediator release experiments was polyclonal goat anti-human IgE which was prepared according to the manufacturer's instructions. The lyophilised powder was reconstituted in 2ml of ultra-pure H2O.
Isolation of basophiles, human lung mast cells and skin mast cellsMixed leucocyte preparations were obtained from whole blood (from healthy and different volunteers) by dextran sedimentation. Briefly 50ml of venous blood was mixed with 12.5ml of 6% dextran and 5ml of 100mM EDTA, and then allowed to sediment for 90min at room temperature. The upper buffy coat layer was removed; cells were recovered by centrifugation (400×g, 8min) and washed twice with PBS. These mixed cell preparations were used in the histamine release experiments. Mast cells were isolated from normal human lung tissue by a modification of the method described by Ali and Pearce (1985). Macroscopically normal tissue from lung resections of patients was obtained with the approval of the Local Research Ethics Committee. The tissue was chopped vigorously for 10min with scissors in a small volume of PBS buffer and then washed over a nylon mesh (100¿m pore size; Incamesh, Warrington, UK) with 0.5–1l of PBS buffer to remove lung macrophages. The tissue was reconstituted in PBS–BSA (10ml per gram of tissue) containing collagenase Ia (0.1mgml−1 of PBS–BSA) and agitated by using a water-driven magnetic stirrer immersed in a water bath set at 37°C. The supernatant was separated from the tissue by filtration over nylon mesh. The collagenase-treated tissue was then reconstituted in a small volume of PBS–BSA buffer and disrupted mechanically with a syringe. The disrupted tissue was then washed over nylon gauze with PBS–BSA. The pooled filtrates were sedimented (400×g, room temperature, 8min), the supernatant discarded and the pellets reconstituted in PBS–BSA (100ml). The pellet was washed twice more. Mast cells were visualised by microscopy using an alcian blue stain. Of the total cells, 3–13% were mast cells. This method generated 2–9×105 mast cells per gram of tissue. Mast cells prepared in this manner were used in mediator release experiments.
MethodsHistamine release experiments were performed in +PBS buffer and assessed in duplicate. Cell suspensions of about 2×104 human mast cells were used per sample. Cells were incubated for either 10 or 15min with the desired concentration of drug in a total volume of, usually, 300¿l and then 30¿l anti-human IgE (1/300 or 1/3000 for mast cells or basophiles, respectively) was added. Histamine release was allowed to proceed for 25 (mast cells) or 45 (basophiles) min at 37°C (Ennis, 1991). Histamine release reactions were terminated by adding 750¿l PBS to all samples. The samples were centrifuged (400×g, 4min, RT) and the histamine present in the cell supernatants was measured by a modification of the automated fluorometric technique of7. Total histamine content of the cells was determined by lysing aliquots of the cells with 1.8% (v/v) perchloric acid. Cells incubated in buffer alone served as a measure of spontaneous histamine release. Histamine release was calculated as a percentage of the total histamine content after subtracting spontaneous histamine release.
Data analysisMaximal responses (Emax) and potencies (pEC50) were determined by non-linear regression analysis (GraphPad Prism, version 3.0a). To determine whether there was any difference in the responses after treatments with drugs, repeated measures analysis of variance was performed.
MaterialsThe following were purchased from the sources indicated; anti-human IgE, aprotinin, BSA, collagenase, dimethyl sulphoxide, DNase, Dowex AG-50W, Dowex AG1-X8, dithiothreitol, HSA, leupeptin, Percoll, phenyl methyl sulphonyl fluoride, soybean trypsin inhibitor, Tween 20, Triton X-100 (all Sigma, Poole, UK); EDTA, calcium chloride and magnesium chloride (BDH, Poole, UK); goat polyclonal anti-human IgE, human serum albumin (HSA), dimethyl suphoxide (DMSO), sodium metabisulphite, rolipram, zaprinast, 3-isobutyl-1-methylxanthine (IBMX), 8-methoxy-methyl-IBMX, theophylline, isoprenaline, N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate (Bu2-cAMP) and N6,2′-O-dibutyrylguanosine 3′,5′-cyclic monophosphate (Bu2-cGMP); Org 30029 (gift from Dr CD Nicholson, Organon, UK); denbufylline (Beecham Pharmaceuticals, UK); 8-Bromo-cAMP, 8-Bromo-cGMP, and siguazodan (Tocris Cookson Ltd., UK); ethanol (BDH, Pool, UK); Ro 20-1724 (Research Biochemicals Incorporated, USA).
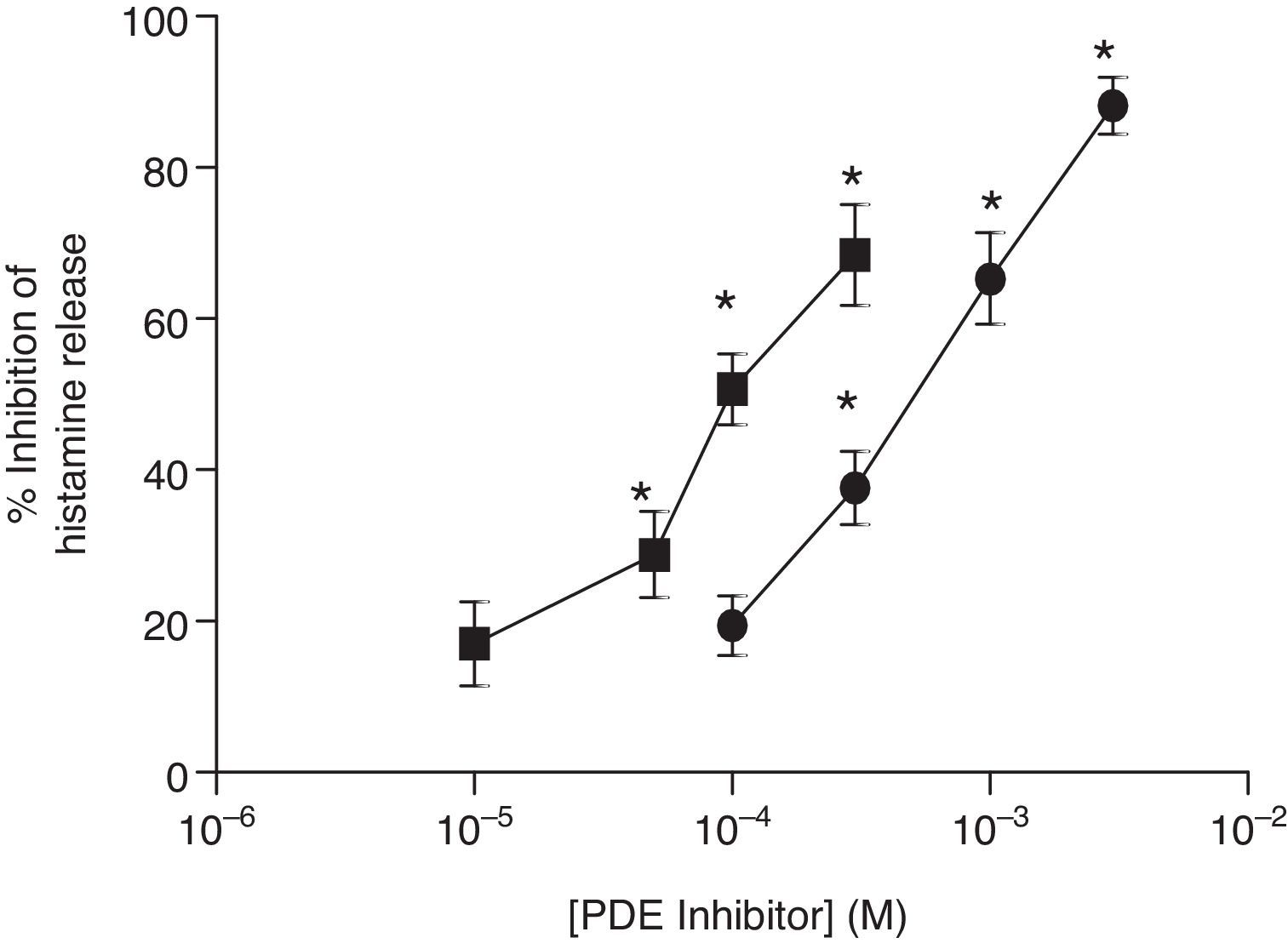
ResultsEffects of PDE inhibitors on IgE-mediated histamine release from skin mast cellsThe effects of non-selective PDE inhibitors, theophylline and IBMX, on histamine release from human skin mast cells (HSMC) were investigated. Cells were incubated with increasing concentrations of PDE inhibitors for 15min before challenge with anti-IgE (1:300). The PDE inhibitors attenuated IgE-mediated histamine release in a dose-dependent manner and to a statistically significant (p<0.05) extent. IBMX was a more potent inhibitor than theophylline of IgE-mediated histamine release in HSMC. EC50 values for the IBMX and theophylline inhibition of histamine release from HSMC were 0.1mM and 1mM, respectively (Fig. 1).
Effect of non-selective PDE inhibitors on histamine release from human skin mast cells (HSMC). The effect of either IBMX (■) or theophylline (●) on the release of histamine from HSMC was determined. Cells were incubated for 15min with a PDE inhibitor and then challenged with an optimal releasing concentration of anti-IgE (1:300) for a further 25min. Results are expressed as the percent inhibition of the control release, which was 19±4%. Statistically significant (p<0.01) levels of inhibition are indicated by asterisks. Values are means±SEM, n=5.
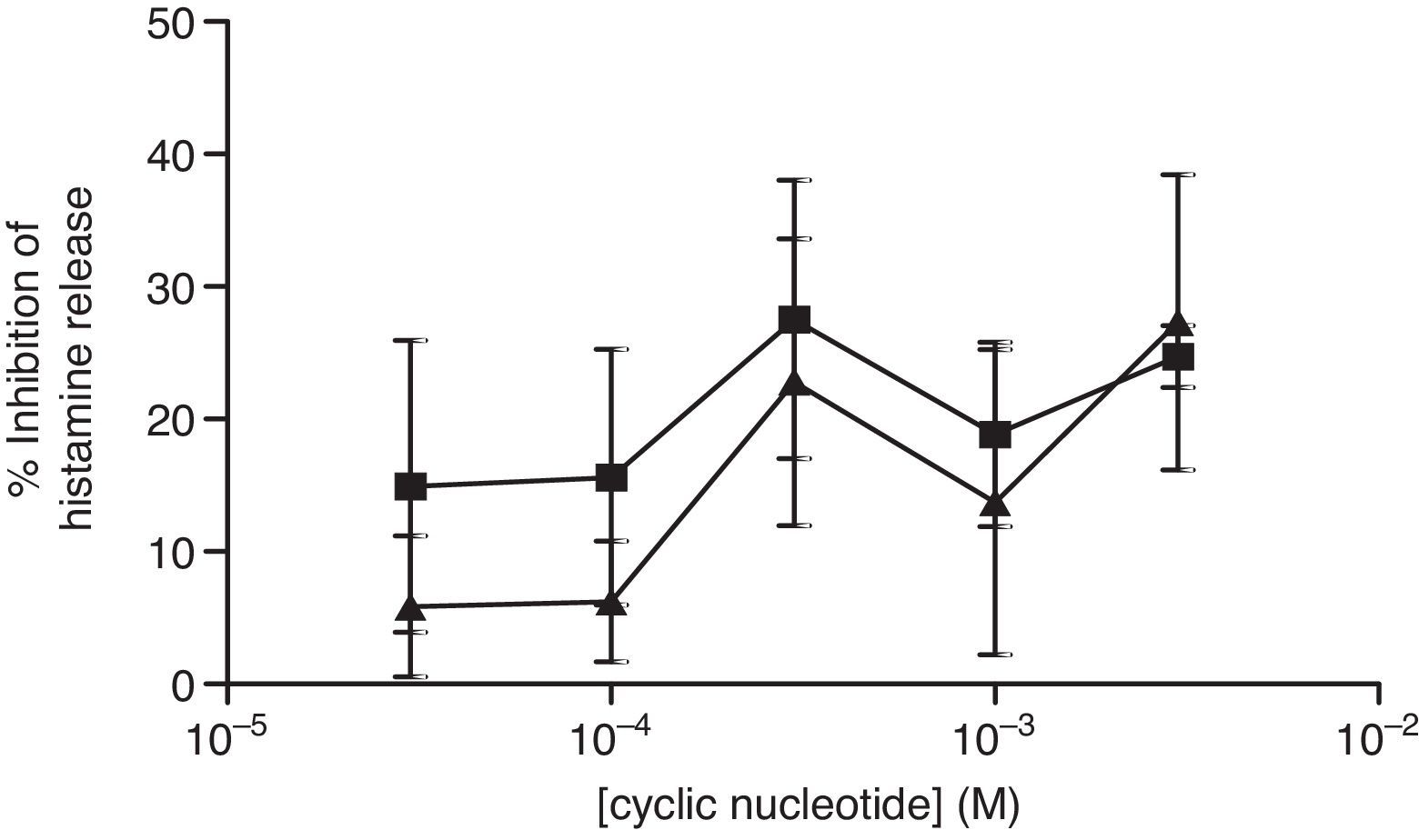
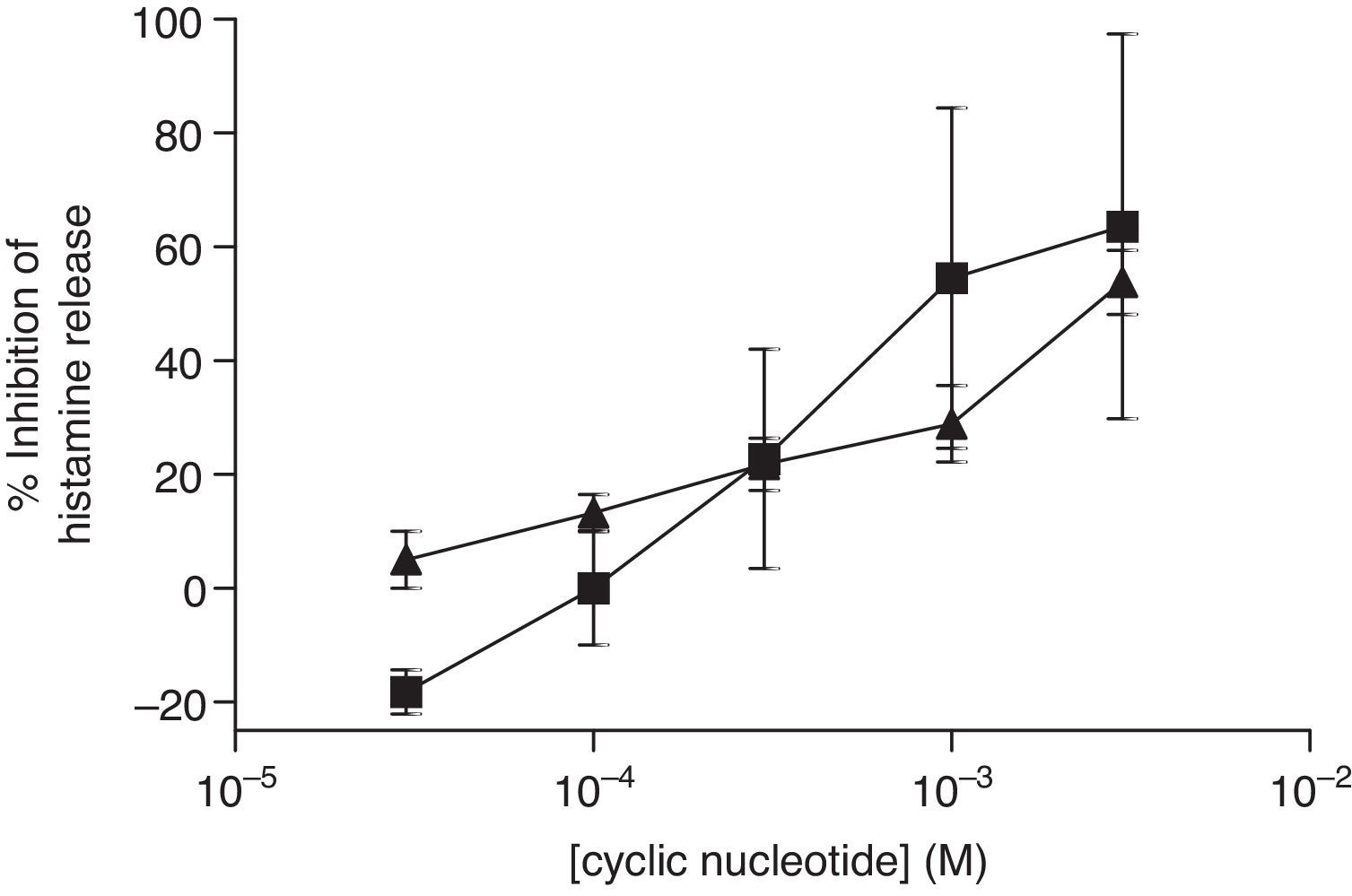
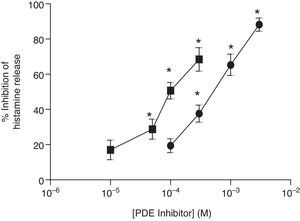
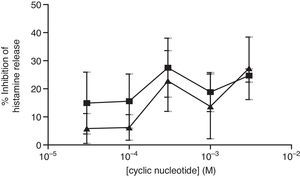
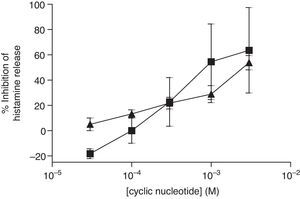
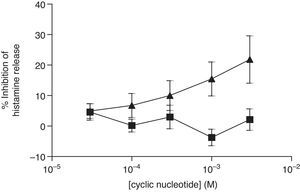
The non-selective inhibitors, IBMX and theophylline, are likely to exert effects on HSMC by elevating cAMP and/or GMP. To investigate the roles of cAMP and cGMP on HSMC the effects of cell-permeant, non-hydrolysable analogues of cAMP and cGMP were studied. The effects of Bu2-cAMP, Bu2-cGMP, 8-Br-cAMP and 8-Br-cGMP on IgE-mediated histamine release from HSMC were studied. Cells were pre-treated for 15min in the presence of increasing concentrations (3×10−5–3×10−3M) of these cyclic nucleotide analogues. Then the cells were triggered with an optimal releasing concentration of anti-IgE (1:300) for a further 25min for the release of histamine. Neither 8-Br-cAMP nor 8-Br-cGMP (Fig. 2) displayed any inhibitory activity on histamine release from HSMC. In further studies the effects of Bu2-cAMP and Bu2-cyclic GMP were investigated. The data show (Fig. 3) both Bu2-cAMP and Bu2-cGMP inhibit histamine release in a dose-dependent manner with maximal inhibitory effects of 63±9% and 53±5%, respectively at 3mM.
Effect of 8-Br-cAMP (■) and 8-Br-cGMP (▴) on human skin mast cells (HSMC). Cells were incubated for 20min with either 8-bromo-cAMP or 8-Br-cGMP before challenge with an optimal releasing concentration of anti-IgE (1:300). Histamine release was allowed to proceed for 25min. Results are presented as the percent inhibition of the control histamine release which was 15±3%. Values are means±SEM, n=6.
Effect of Bu2-cAMP (■) and Bu2-cGMP (▴) on human skin mast cells (HSMC). Cells were incubated for 20min with either Bu2-cAMP or Bu2-cGMP before challenge with an optimal releasing concentration of anti-IgE (1:300). Histamine release was allowed to proceed for 25min. Results are presented as the percent inhibition of the control histamine release which was 18±2%. Values are means±SEM, n=10.
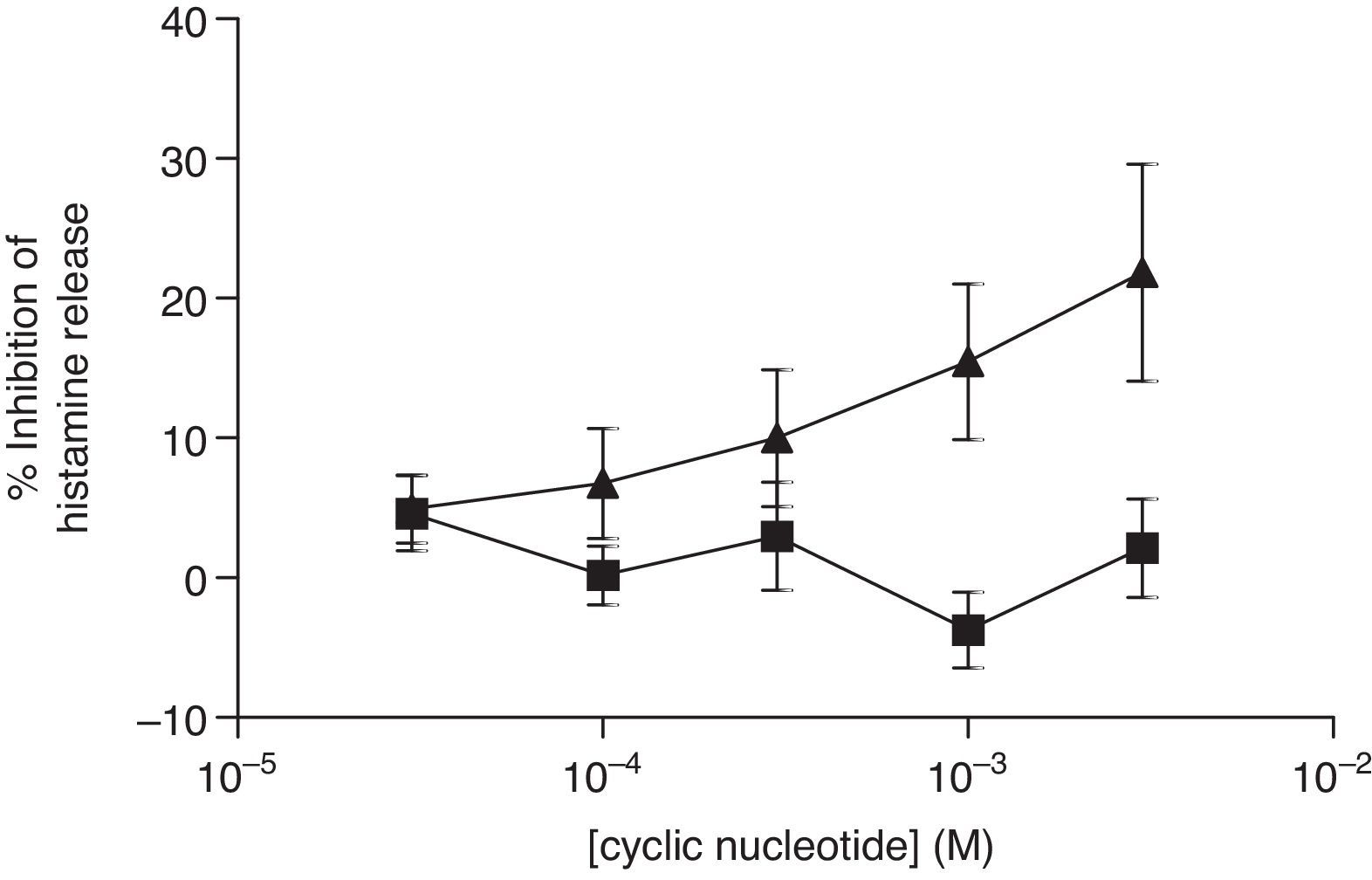
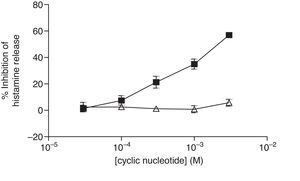
For comparative reasons, the effects of these analogues in HLMC were investigated. Neither 8-Br-cAMP nor 8-Br-cGMP had an inhibitory effect on HLMC (Fig. 4), whereas Bu2-cAMP, but not Bu2-cGMP, inhibited the stimulated release of histamine from HLMC (Fig. 5).
Effect of 8-Br-cAMP (■) and 8-Br-cGMP (▴) on human lung mast cells (HLMC). Cells were incubated for 20min with either 8-Br-cAMP or 8-Br-cGMP before challenge with an optimal releasing concentration of anti-IgE (1:300). Histamine release was allowed to proceed for 25min. Results are presented as the percent inhibition of the control histamine release which was 38±3%. Values are means±SEM, n=3.
Effect of Bu2-cAMP (■) and Bu2-cGMP (△) on human lung mast cells (HLMC). Cells were incubated for 20min with either Bu2-cAMP or Bu2-cGMP before challenge with an optimal releasing concentration of anti-IgE (1:300). Histamine release was allowed to proceed for 25min. Results are presented as the percent inhibition of the control histamine release which was 43±8%. Values are means±SEM, n=4.
Because PDE 4 is the predominant isoform in inflammatory cells,8,9 the effects of the PDE 4-selective inhibitor, rolipram, on IgE-mediated histamine release from HSMC were studied. Cells were pre-treated for 15min with increasing concentrations of rolipram (10−10–10−5M) or DMSO (vehicle) then triggered with an optimal releasing concentration of anti-IgE (1:300) for a further 25min for histamine release. Rolipram (and DMSO over the same equivalent vehicle concentration range of rolipram) failed to inhibit histamine release from HSMC (data not shown).
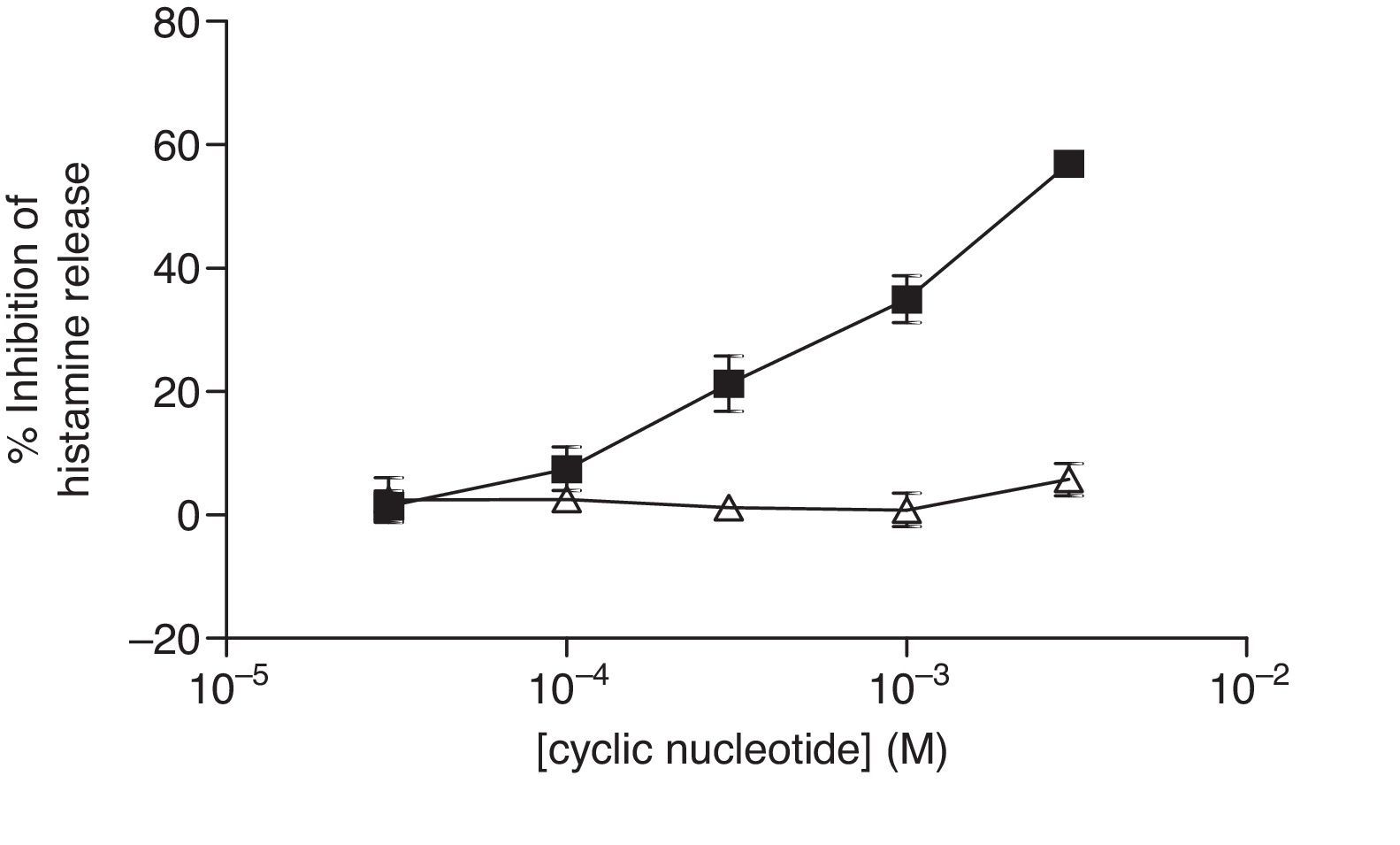
The inability of rolipram to stabilise HSMC responses prompted us to investigate the effects of a wide range of selective PDE inhibitors on the IgE-mediated histamine release from HSMC. Cells were pre-treated for 15min with these agents and then challenged with an optimal releasing concentration of anti IgE (1:300) for a further 25min for the release of histamine. The data show (Table 1) that all the selective PDE inhibitor compounds (10−5M) were ineffective whereas the non-selective PDE inhibitor, theophylline (10−3M), inhibited histamine release from HSMC (74±4% inhibition; p<0.05).
Effects of selective PDE inhibitors on histamine release from human skin mast cells (HSMC), human lung mast cells (HLMC), and human peripheral blood basophils.
| PDE inhibitor class | % Inhibition | ||
|---|---|---|---|
| HSMC | HLMC | Basophiles | |
| 8-Me-IBMX (1) | 16.0±4.9 | 9.4±4.1 | 6.9±2.7 |
| Siguazodan (3) | 20.3±4.9 | 9.5±2 | 5.0±5.5 |
| Org 30029 (3/4) | 0.9±7.2 | 9.3±3 | 45.9±1.8* |
| Rolipram (4) | 23.3±6.2 | 10.2±4.5 | 67.5±2.2* |
| Denbufylline (4) | 20.1±5 | 11.5±2.7 | 49.3±1.0* |
| Ro-2017 (4) | 17.5±6 | 9.5±5.3 | 40.9±2.4* |
| Zaprinast (5) | 13.3±5.5 | 9.5±3 | 10.6±8.8 |
| Theophylline N.S. | 74.2±4.2* | 71.3±4.1* | 77.8±2.1* |
For comparative purposes, the effects of these same compounds on the IgE-mediated release of histamine from both HLMC and basophiles were investigated (Table 1). None of the selective PDE inhibitors had any effect on histamine release from HLMC whereas, in basophiles, compounds with activity at PDE 4 (rolipram, denbufylline, Ro-2017, Org 30029) were effective inhibitors of histamine release.
DiscussionThe results of the present study show that theophylline and IBMX act as effective inhibitors of IgE-mediated histamine release from HSMC. These results suggest that inhibition of PDE can lead to the reduction of the secretory response in skin mast cells. Inhibition of PDE would be expected to cause increases in cyclic nucleotides. Alternative studies indicate that treatment of HLMC with IBMX causes intracellular elevations in cyclic AMP10 although, the effect that non-selective PDE inhibitors have on cGMP content in human mast cells is not known.
In order to gain a better understanding of the roles of cGMP and cAMP in HSMC, we investigated the effects of cell-permeant and non-hydrolysable analogues of these cyclic nucleotides. Neither 8-Br-cAMP nor 8-Br-cGMP had any effects in HSMC. Indeed, to determine whether this was a finding restricted to HSMC, the effects of these analogues on HLMC were investigated. These analogues were ineffective on these cells and further studies from our group11 have shown that 8-bromo derivatives are ineffective in basophiles. This could be because the 8-bromo derivatives are not very cell-permeant. Therefore, we decided to look at alternative analogues Bu2-cAMP and Bu2-cGMP. Both analogues were effective inhibitors of histamine release from HSMC, suggesting that both cAMP and cGMP may inhibit the responses of HSMC. However, caution is needed with this interpretation as dibutyryl compounds can be cleaved intracellulary to dibutyrate which is known to affect cell function. However, arguing against an effect of butyrate on HSMC is the finding that Bu2-cAMP, and not Bu2-cGMP, inhibited histamine release from HLMC.
In further studies the effects of selective PDE inhibitors on HSMC were investigated. None of the inhibitors tested had any effect on the IgE-mediated release of histamine. Interestingly, therefore, HSMC behave much like HLMC which are also insensitive to PDE 4 inhibitors (8). These findings suggest that the PDE that regulates HSMC and HLMC function is something other than PDE 1, 3, 4, or 5. Mast cells, therefore, differ from the vast majority of inflammatory cells which are known to be regulated by PDE 48,9. This includes the human basophile, which the present study has shown to be particularly sensitive to inhibitors of PDE4, confirming previous studies9,11. It is possible that the methods used to prepare HSMC or that the maturation state of the isolated mast cells which were obtained from the foreskins of neonates and infants could influence the expression of PDEs. This issue could be resolved by isolating mast cells from adult skin. However, that HLMC are also refractory to PDE4 inhibitors makes it probable that human mast cells, in general, do not express PDE4.
The emphasis of the present study was to characterise PDE isoforms in HSMC. The data suggest that unlike most inflammatory cells, PDE4-selective inhibitors are ineffective stabilisers of HSMC activity. In this regard HSMC are similar to HLMC. However, due to the functional heterogeneity displayed by mast cells the possibility exists that the responses to PDE inhibitors of HSMC and HLMC may not necessarily reflect those of alternative subsets of mast cells. In summary, the present work has shown that although those PDEs appear to regulate HSMC, the nature of this PDE (s) remains uncertain.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestThe authors have no conflict of interest to declare.