Food allergy and respiratory allergy are two frequently associated diseases and with an increasing prevalence. Several reports show the presence of respiratory symptoms in patients with food allergy, while certain foods may be related to the development or exacerbation of allergic rhinitis and asthma.
The present update focuses on this relationship, revealing a pathogenic and clinical association between food and respiratory allergy. This association is even more intense when the food hypersensitivity is persistent or starts in the early years of life. Food allergy usually precedes respiratory allergy and may be a risk factor for allergic rhinitis and asthma, becoming a relevant clinical marker for severe atopic asthma. Furthermore, the presence of co-existing asthma may enhance life-threatening symptoms occurring during a food allergic reaction.
Recommendations for dietary restrictions during pregnancy and breastfeeding to prevent the development of respiratory allergy are controversial and not supported by consistent scientific data. Current recommendations from medical societies propose exclusive breastfeeding during the first four months of life, with the introduction of solid food in the fourth to the seventh month period of life. A delayed introduction of solid food after this period may increase the risk of developing subsequent allergic conditions.
Further studies are encouraged to avoid unjustified recommendations involving useless dietary restrictions.
The relationship between food allergy and respiratory allergy is included in the concept of atopic march,1 which states that various manifestations of allergic disorders are closely related. They usually begin with atopic dermatitis, progressing with the development of food allergy and subsequently favouring the occurrence of allergic rhinitis and asthma.2 Food allergy usually precedes hypersensitivity to aeroallergen, sharing a common mechanism which involves a specific immunoglobulin (Ig) E capable of releasing inflammatory mediators.
Following the concept of a single united airway which combines the upper and lower respiratory tract, we focus on the association between food allergy and respiratory symptoms, including data concerning the involvement of nasal and bronchial mucosa. We also include the middle ear, which may be targeted in the allergic response through a Th2 response with a local IgE production and the releasing of mast cell mediators3. In addition to these local inflammatory processes, a nasopharyngeal oedema occluding the proximal portion of the tube may also be present, with a remarkable change in the tubal functionality.4
Studies on genetics and epidemiology have demonstrated a close relationship between allergic rhinitis and asthma5. The Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines promoted in 2001 by the World Health Organization (WHO) and updated in 2008, recognise the importance of this association.6 Around 80–95% of patients with allergic asthma have allergic rhinitis7 and about 40% of patients with allergic rhinitis develop asthmatic symptoms, being rhinitis a risk factor for asthma.8
The objective of this paper has been to review the epidemiology and clinical association between respiratory and food allergy, also updating the recommendations on dietary restriction during pregnancy and breastfeeding, and the timing of introduction of solid foods into the infant's diet as measures to reduce the risk to develop respiratory allergy.
Methods and critical assessment of studiesSearch strategyIn order to perform the search for the most suitable and representative articles for each of the topics to be addressed, two allergists with extensive clinical experience in respiratory and food allergy were assembled with the objective of updating the conclusions of the articles. Then, they carried out a comprehensive bibliographic search selecting scientific articles until 2014.
The search was performed through PubMed, a comprehensive database of biomedical literature which allows access to MEDLINE journals and biological sciences books, using different combinations of the following keywords in order to optimise the bibliographic search: food allergy, allergic rhinitis, allergic asthma, respiratory allergy, breastfeeding, solid foods, prevalence and epidemiology. These words have also remained as the keywords of this review.
Inclusion criteriaUsing the terms selected, more than 300 articles were found on PubMed. Those potential useful papers were initially selected according to their title and data collected in the summary. Out of them, 197 were categorised as potentially relevant and published in English, Spanish or French language, including:
- -
Meta-analysis, reviews, consensus statement and position papers that would include any related information to the subject. They were considered as interesting even if the relationship was only collateral.
- -
Studies with statistically significant results regardless of their sample size, as well as studies with a suitable population size (n>200) and including enough data about the methodology used, allowing us to identify potential biases.
Those articles rated as potentially interesting were thoroughly read by the two main authors to obtain more detailed information, and they were classified as included, not included and unsure according to a given assessment, ruling out less relevant studies.
The final study sample comprised 104 articles that were strictly evaluated by the main authors, identifying the necessary information on methodology, results and conclusions. With all these data, they developed a first draft, in which the information was classified as very relevant, relevant or less relevant; they also tried to identify contradictions between different articles. This first draft was reviewed by another four undersigned authors, and, as a result, a second draft was written. This new manuscript was evaluated by all the remaining authors, and then they could suggest modifications. When scientific evidence was considered as insufficient, doubts were discussed and decisions taken in order to agree on the most adequate approach. The final version of the document was accorded and reviewed by the whole study group. The most relevant studies are summarised in Table 1.
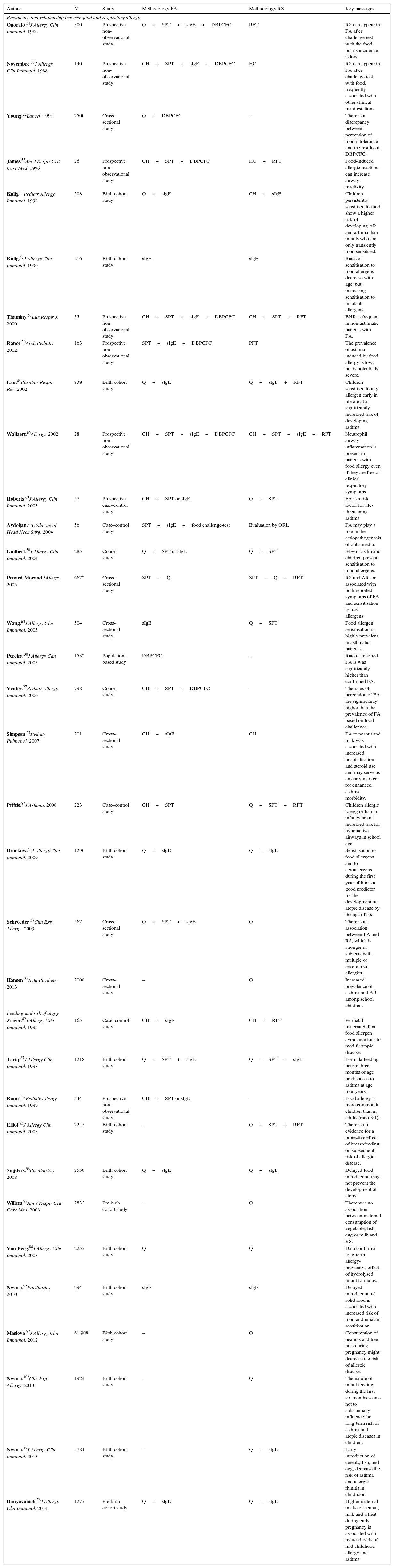
The most relevant studies and key messages.
| Author | N | Study | Methodology FA | Methodology RS | Key messages |
|---|---|---|---|---|---|
| Prevalence and relationship between food and respiratory allergy | |||||
| Onorato.54J Allergy Clin Immunol. 1986 | 300 | Prospective non-observational study | Q+SPT+sIgE+DBPCFC | RFT | RS can appear in FA after challenge-test with the food, but its incidence is low. |
| Novembre.55J Allergy Clin Immunol. 1988 | 140 | Prospective non-observational study | CH+SPT+sIgE+DBPCFC | HC | RS can appear in FA after challenge-test with food, frequently associated with other clinical manifestations. |
| Young.22Lancet. 1994 | 7500 | Cross-sectional study | Q+DBPCFC | – | There is a discrepancy between perception of food intolerance and the results of DBPCFC. |
| James.53Am J Respir Crit Care Med. 1996 | 26 | Prospective non-observational study | CH+SPT+DBPCFC | HC+RFT | Food-induced allergic reactions can increase airway reactivity. |
| Kulig.44Pediatr Allergy Immunol. 1998 | 508 | Birth cohort study | Q+sIgE | CH+sIgE | Children persistently sensitised to food show a higher risk of developing AR and asthma than infants who are only transiently food sensitised. |
| Kulig.47J Allergy Clin Immunol. 1999 | 216 | Birth cohort study | sIgE | sIgE | Rates of sensitisation to food allergens decrease with age, but increasing sensitisation to inhalant allergens. |
| Thaminy.65Eur Respir J. 2000 | 35 | Prospective non-observational study | CH+SPT+sIgE+DBPCFC | CH+SPT+RFT | BHR is frequent in non-asthmatic patients with FA. |
| Rancé.56Arch Pediatr. 2002 | 163 | Prospective non-observational study | SPT+sIgE+DBPCFC | PFT | The prevalence of asthma induced by food allergy is low, but is potentially severe. |
| Lau.45Paediatr Respir Rev. 2002 | 939 | Birth cohort study | Q+sIgE | Q+sIgE+RFT | Children sensitised to any allergen early in life are at a significantly increased risk of developing asthma. |
| Wallaert.66Allergy. 2002 | 28 | Prospective non-observational study | CH+SPT+sIgE+DBPCFC | CH+SPT+sIgE+RFT | Neutrophil airway inflammation is present in patients with food allergy even if they are free of clinical respiratory symptoms. |
| Roberts.69J Allergy Clin Immunol. 2003 | 57 | Prospective case–control study | CH+SPT or sIgE | Q+SPT | FA is a risk factor for life-threatening asthma. |
| Aydoğan.72Otolaryngol Head Neck Surg. 2004 | 56 | Case–control study | SPT+sIgE+food challenge-test | Evaluation by ORL | FA may play a role in the aetiopathogenesis of otitis media. |
| Guilbert.50J Allergy Clin Immunol. 2004 | 285 | Cohort study | Q+SPT or sIgE | Q+SPT | 34% of asthmatic children present sensitisation to food allergens. |
| Penard-Morand.2Allergy. 2005 | 6672 | Cross-sectional study | SPT+Q | SPT+Q+RFT | RS and AR are associated with both reported symptoms of FA and sensitisation to food allergens. |
| Wang.63J Allergy Clin Immunol. 2005 | 504 | Cross-sectional study | sIgE | Q+SPT | Food allergen sensitisation is highly prevalent in asthmatic patients. |
| Pereira.30J Allergy Clin Immunol. 2005 | 1532 | Population-based study | DBPCFC | – | Rate of reported FA is was significantly higher than confirmed FA. |
| Venter.27Pediatr Allergy Immunol. 2006 | 798 | Cohort study | CH+SPT+DBPCFC | – | The rates of perception of FA are significantly higher than the prevalence of FA based on food challenges. |
| Simpson.64Pediatr Pulmonol. 2007 | 201 | Cross-sectional study | CH+sIgE | CH | FA to peanut and milk was associated with increased hospitalisation and steroid use and may serve as an early marker for enhanced asthma morbidity. |
| Priftis.57J Asthma. 2008 | 223 | Case–control study | CH+SPT | Q+SPT+RFT | Children allergic to egg or fish in infancy are at increased risk for hyperactive airways in school age. |
| Brockow.43J Allergy Clin Immunol. 2009 | 1290 | Birth cohort study | Q+sIgE | Q+sIgE | Sensitisation to food allergens and to aeroallergens during the first year of life is a good predictor for the development of atopic disease by the age of six. |
| Schroeder.37Clin Exp Allergy. 2009 | 567 | Cross-sectional study | Q+SPT+sIgE | Q | There is an association between FA and RS, which is stronger in subjects with multiple or severe food allergies. |
| Hansen.35Acta Paediatr. 2013 | 2008 | Cross-sectional study | – | Q | Increased prevalence of asthma and AR among school children. |
| Feeding and risk of atopy | |||||
| Zeiger.42J Allergy Clin Immunol. 1995 | 165 | Case–control study | CH+sIgE | CH+RFT | Perinatal maternal/infant food allergen avoidance fails to modify atopic disease. |
| Tariq.87J Allergy Clin Immunol. 1998 | 1218 | Birth cohort study | Q+SPT+sIgE | Q+SPT+sIgE | Formula feeding before three months of age predisposes to asthma at age four years. |
| Rancé.32Pediatr Allergy Immunol. 1999 | 544 | Prospective non-observational study | CH+SPT or sIgE | – | Food allergy is more common in children than in adults (ratio 3:1). |
| Elliot.85J Allergy Clin Immunol. 2008 | 7245 | Birth cohort study | – | Q+SPT+RFT | There is no evidence for a protective effect of breast-feeding on subsequent risk of allergic disease. |
| Snijders.96Paediatrics. 2008 | 2558 | Birth cohort study | Q+sIgE | Q+sIgE | Delayed food introduction may not prevent the development of atopy. |
| Willers.75Am J Respir Crit Care Med. 2008 | 2832 | Pre-birth cohort study | – | Q | There was no association between maternal consumption of vegetable, fish, egg or milk and RS. |
| Von Berg.84J Allergy Clin Immunol. 2008 | 2252 | Birth cohort study | Q | Q | Data confirm a long-term allergy-preventive effect of hydrolysed infant formulas. |
| Nwaru.95Paediatrics. 2010 | 994 | Birth cohort study | sIgE | sIgE | Delayed introduction of solid food is associated with increased risk of food and inhalant sensitisation. |
| Maslova.77J Allergy Clin Immunol. 2012 | 61,908 | Birth cohort study | – | Q | Consumption of peanuts and tree nuts during pregnancy might decrease the risk of allergic disease. |
| Nwaru.102Clin Exp Allergy. 2013 | 1924 | Birth cohort study | – | Q | The nature of infant feeding during the first six months seems not to substantially influence the long-term risk of asthma and atopic diseases in children. |
| Nwaru.12J Allergy Clin Immunol. 2013 | 3781 | Birth cohort study | – | Q+sIgE | Early introduction of cereals, fish, and egg, decrease the risk of asthma and allergic rhinitis in childhood. |
| Bunyavanich.76J Allergy Clin Immunol. 2014 | 1277 | Pre-birth cohort study | Q+sIgE | Q+sIgE | Higher maternal intake of peanut, milk and wheat during early pregnancy is associated with reduced odds of mid-childhood allergy and asthma. |
Abbreviations: AR: allergic rhinitis; BHR: bronchial hyperreactivity; CH: clinical history; DBPCFC: double-blind, placebo-controlled food challenge; FA: food allergy; N: sample size; Q: questionnaire; RFT: respiratory functional test; RS: respiratory symptoms; SBPCFC: single-blind, placebo-controlled food challenge; sIgE: specific IgE; SPT: skin prick-test.
As a result of our assessment, it may be noteworthy that several findings reported as relevant in most of the studies are really questionable and therefore, the preventive measures based on them could also be unnecessary, ineffective and costly in many cases.
The main gap presented in the evaluated studies was the lack of a rigorous design, leading to multiple errors and biases, some of which are detailed below:
- 1
Selection bias, with the study group lacking adequate sample size, homogeneity and age stratification.9
- 2
Reverse causality bias, which commonly occurs in observational studies: families with children with early signs of allergy or a family history of allergy can voluntarily modify their children's diet in an attempt to decrease the risk of developing allergic diseases, extending duration of breastfeeding and delaying the introduction of solid foods. This might suggest the false evidence of a temporal relationship between the introduction of solid foods and the subsequent development of allergy, which is really more influenced by the family atopic burden than by the time of the introduction of food itself.10
- 3
Cross-sectional studies have been used to assess prevalence, but also to assess causality. Instead, prospective longitudinal cohort studies, although difficult in design, may be warranted.11
- 4
Most of the studies performed about breastfeeding and the introduction of solid foods in the diet have been observational, which certainly increases the number of biases.12 Randomised prospective epidemiological studies should have been conducted in order to reach accurate conclusions, although it is known that this type of studies can generate ethical conflicts because the children's nutrition could be modified.
- 5
Information about food and respiratory allergy has often been based on questionnaires. Despite having been validated for epidemiological studies in allergic diseases, even nationally13 or internationally, such as the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire,14 they may induce biases difficult to control: scant unification in the different definitions used, differences to complete the questionnaire (non-response bias),15 recall bias in data collection16 or dependence of the educational level of parents are frequently found.
- 6
Most of the studies use the terms sensitisation and allergy interchangeably, regardless of the fact that detection of hypersensitivity to an allergen is not equivalent to a clinical diagnosis of allergy, unless there is correlation with clinical data.17 Sensitisation may be a normal, harmless, and transitory phenomenon, which does not necessarily correlate with allergic disease.
- 7
Diagnosis of food allergy based exclusively on skin tests or specific IgE is questionable. Although the negative predictive value of these tests is 95%,18 their positive predictive value drops to a level below 50%.19 An oral challenge test with the offending food is the gold standard for the diagnosis of food allergy20 and when controlled challenge-tests have been performed, the diagnosis of food allergy decreases to half of those patients with positive skin tests.21 In cases of self-reported food allergy, it can only be confirmed in less than 20% after challenge-test.22
Although single-blind challenge-test could be accepted in clinical and epidemiological studies,23 a double-blind placebo-controlled challenge-test is mandatory to avoid the observer's bias.24 It is noteworthy that, in many of the studies included in this paper, the diagnosis of food allergy was not confirmed by a challenge-test.
- 8
Most studies were performed in developed countries, and the epidemiological data obtained are difficult to extrapolate to other groups with different characteristics, not only in the genetic predisposition,19 but also in their relation to dietary habits and environmental exposures,25 which could be highly variable among different populations.
- 9
It is outstanding that only a few studies have controlled potential confounding factors by the use of multivariate analysis.26
- 10
Finally, the weakness of the reviewed studies did not allow us to establish any updated conclusion with a high grade of recommendation, and therefore the recommendations previously reported about the comparative benefits and risks of different prevention approaches should be revised.
A remarkable increase in the prevalence of both entities has been notified, as happens with all types of allergic diseases.
Prevalence of food allergyThe prevalence of food allergy is difficult to estimate. In any case, the rate of perception of having food allergy by the patient is greater than the prevalence of sensitisation to food allergens, and this difference is even greater when compared to the true prevalence of food allergy based on a food challenge-test.27 Some studies, especially those based on questionnaires, may also overestimate food allergy, recording symptoms of non-allergic intolerance.28
A meta-analysis by Rona et al.,29 which evaluated fifty-one previous studies, found an overall prevalence of food allergy ranging from 3% to 35%. The same authors, focused on six studies with a diagnosis based on an oral challenge-test, obtained a three-time smaller prevalence, ranging from 1% to 10.8%.
Pereira et al. in a cohort study confirmed the variability in the results according to the diagnostic method. The prevalence was assessed in two populations of different ages: in 11-year-old children, a percentage of 11.6% of reported food allergy and 5.1% of food sensitisation determined by skin testing were obtained, with a remarkable decrease to a 1% after a single-blind oral challenge-test and to 0.1% if the challenge was double-blinded. Similar percentages were obtained in a 15-year-old population, with percentages of 12.4%, 4.9%, 1% and 0.5%, respectively.30
A recent meta-analysis by the European Academy of Allergology and Clinical Immunology (EAACI) Food Allergy and Anaphylaxis Guidelines Group31 published in 2014 concluded that the prevalence of self-reported food allergy in Europe is 6%, decreasing to levels below 1% when allergy was confirmed by food challenge-test. The same study confirms that the prevalence has been increasing in recent years, while the incidence remains at stable levels.
In summary, and according to the previous considerations, the most reliable data indicate that the prevalence of food allergy in the general population ranges from 2% to 6% in children, with a ratio of children to adults of 3:1.32
Prevalence of respiratory allergy [rhinitis and asthma]Prevalence of both diseases has increased, not only due to improvements in the screening and diagnostic methods, but also to environmental exposure factors. Among these, changes in dietary habits, which could promote the exposure to novel food antigens and enable new sensitivities and respiratory symptoms, may be included.33
The prevalence of respiratory diseases in the U.S. increased by 75% between 1980 and 1994, reaching up to a 160% in children under 5 years old.34 A more recent series from Norway assessed the prevalence of both diseases between 1985 and 2008 in 7–14 year-old schoolchildren, reporting an increase in asthma prevalence from 7.3 to 17.6% and in allergic rhinitis from 15.9 to 24.5%.35 Updated data collected in 2013 in the third phase of the ISAAC study showed 14.1% for asthma and 14.6% for rhinoconjunctivitis prevalence in adolescents, and 11.7% for asthma and 8.5% for rhinoconjunctivitis in 6 and 7 year-old children.36
Association between food allergy and respiratory allergyMany data have been reported suggesting a strong association between food and respiratory allergy.37 Unfortunately, they have been mainly obtained from cross-sectional studies with multiple methodological biases, and therefore, the magnitude of the association between the two entities could be questioned. There are only a few studies with a correct design including a proper and confirmed diagnosis, but their results are limited due to small sample size.38
Further prospective longitudinal cohort studies with the appropriate size including a follow-up from birth (birth cohort) are needed to validate these data, besides investigations to clarify common risk factors that, in addition to atopy, may underlie the association between food allergy and respiratory allergy.
EpidemiologyFood allergy and respiratory diseases frequently coexist: food allergy is considered a risk factor for the development of some other allergic diseases. A temporal relationship between the two conditions has also been confirmed,39 and the occurrence of food allergy leads the way to the development of allergic rhinitis and asthma.38 It has been reported that 29% of children with food allergy are asthmatic40 and that 8% of asthmatic children have food allergy.41
Children with food allergies have atopic manifestations such as asthma, eczema or respiratory allergies with a frequency 2–4 times higher compared to those without food allergies: 77.4% of food-allergic children at seven years of age developed nasal or bronchial symptoms, while these respiratory symptoms were only present in 45.5% of non-allergic children.42
The risk of developing subsequent atopic manifestations is greater if the food hypersensitivity arises in the early years of life.43 This risk is also increased with persistent food allergy [longer than one year], showing a 3.4 times greater risk of developing allergic rhinitis44 and a 5.5 greater risk of developing asthma45 compared to those with transient food hypersensitivity. Some other risk factors include the presence of a larger number of food sensitisations or showing a more severe food allergy.46
The basis for the association between food allergy and respiratory allergy is not well studied. Both conditions share some risk factors such as atopy, and asymptomatic sensitisation to food allergens is frequently the first step for the subsequent development of atopic diseases.47 This food sensitisation could also facilitate the hypersensitivity to aeroallergens, which is indeed the biggest risk factor for developing asthma and allergic rhinitis. It has been reported that children with positive specific IgE antibodies to egg in the first year of life have a strong risk of sensitisation to aeroallergens by the age of three years compared to non-sensitised children, as well as an increased risk of developing respiratory disease later in life.48
Sensitisation to egg, peanut and milk has been postulated as a marker for the development of symptoms of rhinitis and asthma and they have been included as a minor criterion in the Asthma Predictive Index (API)49 as amended by Guilbert et al.50 to evaluate children with recurrent wheezing. But, in this regard, we note that additional studies46 emphasise that only clinically relevant food allergy shows a consistent association with respiratory allergy, while a simple food sensitisation is not associated.
Pathogenic and clinical aspectsAccording to the concept that all the mucosal immune system is involved in allergic diseases, food allergy can elicit symptoms affecting different organs and systems, with the skin and the gastrointestinal tract being the most commonly associated. Respiratory symptoms are less frequent, but their presence indicates greater severity of food allergies, and often occurring in the context of anaphylaxis. The presence of isolated symptoms like rhinitis or asthma as the only manifestation of a food allergy is exceptional.51
The presence of mucus with gastric content in the airways due to microaspirations related to gastro-oesophageal reflux has been mentioned as a sensitisation mechanism.52 The aspirated food allergens may sensitise the T cells localised in these tissues, favouring the production of specific IgE against food. Subsequent ingestion of these foods could induce the release of mast cell mediators by means of an IgE-dependent mechanism, as well as the activation of lymphoid cells and eosinophils in the airway mucosa, thus contributing to the inflammation of the airway.53
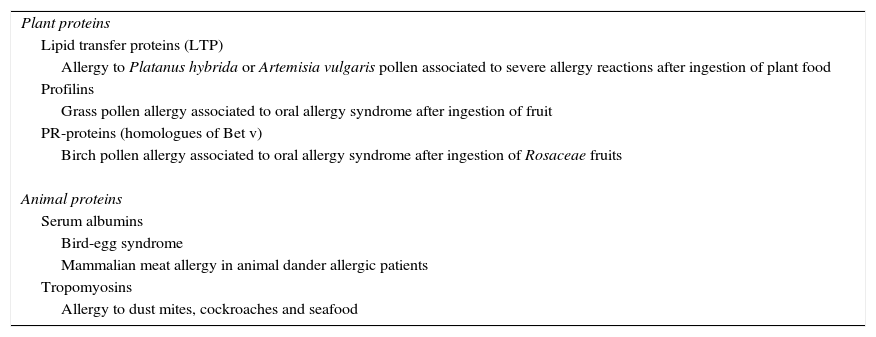
The association between food allergy and respiratory allergy has been confirmed in well-defined syndromes in which patients experience symptoms due to sensitisation to antigens, named panallergens, present simultaneously in foods and aeroallergens, which are responsible for cross-reactivity among allergens (Table 2).
Syndromes associated with allergy to both aeroallergens and food allergens.
| Plant proteins |
| Lipid transfer proteins (LTP) |
| Allergy to Platanus hybrida or Artemisia vulgaris pollen associated to severe allergy reactions after ingestion of plant food |
| Profilins |
| Grass pollen allergy associated to oral allergy syndrome after ingestion of fruit |
| PR-proteins (homologues of Bet v) |
| Birch pollen allergy associated to oral allergy syndrome after ingestion of Rosaceae fruits |
| Animal proteins |
| Serum albumins |
| Bird-egg syndrome |
| Mammalian meat allergy in animal dander allergic patients |
| Tropomyosins |
| Allergy to dust mites, cockroaches and seafood |
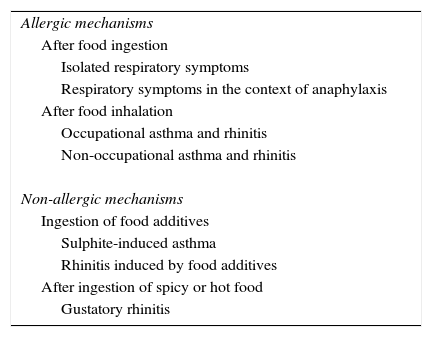
The severity of symptoms is highly variable and can be caused by either allergic or non-allergic mechanisms and triggered after the ingestion or inhalation of foods (Table 3).
Pathogenic mechanisms described in respiratory allergy (rhinitis and asthma) associated with food allergy.
| Allergic mechanisms |
| After food ingestion |
| Isolated respiratory symptoms |
| Respiratory symptoms in the context of anaphylaxis |
| After food inhalation |
| Occupational asthma and rhinitis |
| Non-occupational asthma and rhinitis |
| Non-allergic mechanisms |
| Ingestion of food additives |
| Sulphite-induced asthma |
| Rhinitis induced by food additives |
| After ingestion of spicy or hot food |
| Gustatory rhinitis |
Classic studies indicated that 2% of asthmatic patients had respiratory symptoms after the ingestion of foods,54 especially among children.55 Subsequent studies have redefined these claims, noting that after a double-blind placebo-controlled oral challenge-test with the offending food in asthmatic children with food allergy, the occurrence of nasal and bronchial symptoms was 6.1% and 9.5% respectively.56 The most frequently involved foods were egg, milk, peanut, soy, fish, shellfish, wheat and nuts,57 with the first three foods being mentioned as responsible for 80% of all the reactions.27
In addition, symptoms of rhinitis and asthma may appear in children after handling foods or in the presence of vapours and fumes during cooking, triggered by the inhalation of volatile food antigens, especially with plant foods, fish and seafood.
Finally, we should consider other non-immunological symptoms of food and additives intolerance, such as asthma due to ingestion of sulphites58, rhinitis due to ingestion of monosodium benzoate, tartrazine, erythrosine and monosodium glutamate or nasal discomfort after eating hot or spicy foods [gustatory rhinitis], that were once related to allergic mechanisms erroneously27.
Rhinitis and food allergyThe presence of rhinitis may promote or aggravate responses to food allergens in the intestinal mucosa. In patients with chronic rhinosinusitis, nasal mucosa and nasal secretions are frequently colonised by Staphylococcus aureus producing staphylococcal enterotoxin B (SEB). This toxin is capable to act as a superantigen stimulating T cells and to modify gastrointestinal homeostasis increasing the intestinal absorption and favouring the occurrence of food allergy.59 This hypothesis has been confirmed in rodents, when the administration of SEB along with a food antigen has promoted IgE-mediated reactivity to food.60
Liu et al. confirmed the importance of this colonisation by Staphylococcus aureus. They performed a clean-up of paranasal sinuses in patients with food allergy and chronic sinusitis. After the surgery, there was a decrease in the load of Staphylococci and therefore, of the production of SEB, obtaining an immunomodulatory effect with the attenuation of the Th2 response to foods. This result was confirmed by the evidence of a decrease in the skin test reactivity to foods and a lower response after an oral challenge-test, leading to a better control of the symptoms of food allergy.61
Asthma and food allergyMany patients report that daily ingestion of certain foods, especially dairy products, make asthma worse,62 but it has only been confirmed in fewer than 5% of patients when studies have been conducted using a food challenge-test with spirometric control.54 A higher risk of worsening respiratory disease and deteriorating asthma control is observed in asthmatic patients when food allergy is confirmed,63 and this risk is even increased when patients are sensitised to more than one food.64
Severe asthma may deteriorate after chronic ingestion of even small amounts of a food to which the patient is allergic. For this reason, those patients with refractory asthma that do not respond to the ordinary treatment, and those with postprandial crisis or with unexplained exacerbations, in addition, to rule out a concomitant gastrointestinal disorder such as gastro-oesophageal reflux, should be systematically questioned about a possible relationship with the intake of foods51 and screened for food allergy.18
Recent studies have shown that non-asthmatic patients with food allergy exhibited a subclinical bronchial hyperactivity after a controlled oral challenge test with the food to which they were allergic. This hyperactivity was confirmed by a methacholine challenge-test,65 showing a predominantly neutrophilic bronchial inflammation66 mediated by the increased secretion of interleukin 8 from activated monocytes of bronchial mucosa.67
On the other hand, asthmatic patients show 14 times more risk of developing more severe symptoms during a reaction to foods68 than non-asthmatic subjects.51 Roberts et al. found that food allergy was an independent risk factor for fatal asthma, with an adjusted OR of 5.89 (95% CI: 1.06–32.61) in patients from one to sixteen years old who required ventilation measures in Paediatric intensive care unit69.
Thus, the occurrence of food allergy may be a phenotypic marker of severe atopic asthma63 and, in turn, asthma would be a risk factor for the appearance of severe symptoms after food intake in asthmatic children and adolescents.70
Otitis and food allergyAlthough a role for food allergy in serous otitis media (SOM) has been proposed, this association is still controversial, suggesting that the presence of IgG complexes to foods could contribute to the occurrence of some symptoms of serous otitis.71 Some studies indicate that 44% of patients with SOM present with food allergies72 and that an exclusion diet of those foods with positive skin tests could improve SOM, reactivating after reintroduction.
Despite these data, well-designed studies are lacking to support this claim and in the meantime, a routine testing for food allergy in children with SOM cannot be recommended.
Could preventive measures in early life modify the atopic march?Previous data indicating a strong association between food allergy and respiratory allergy could suggest that an early intervention in the diet during pregnancy, breastfeeding and the introduction of solid foods could decrease the risk of asthma and rhinitis by preventing the sensitisation to food antigens.73 As described above, the conclusions obtained in different studies are contradictory.
Feeding in pregnancy and risk of atopyThe American Academy of Paediatrics (AAP) recommended in 2000 that mothers at high risk of family atopy should eliminate foods like nuts during pregnancy.74 However, later studies have noted that the intake of eggs, milk or fish in the last month of pregnancy was not associated with an increased childhood asthma development later in life.75 Even milk intake during the first trimester of pregnancy76 and nut intake during midpregnancy77 were associated with decreased odds for childhood asthma and allergic rhinitis in the mid-childhood.
A recent meta-analysis78 found that dietary restriction of potentially sensitising foods during pregnancy, even in high-risk families, cannot substantially reduce the risk of atopic diseases in children, and in any case, if any type of protection were achieved, it would be of limited duration.79 In addition, these restrictions may adversely affect both foetal and maternal nutrition with a decreased gestational weight gain and a low birth weight.
Breastfeeding and risk of atopyThe role of maternal breastfeeding as a protective factor against the development of respiratory diseases has been highly controversial, and its recognised protective effect to reduce infections and respiratory complications in childhood may erroneously suggest a similar protection of breastfeeding to prevent allergic disease.
Several authors have claimed for the preventive role of maternal breastfeeding, noting that breastfeeding maintained for at least three months is associated with a low prevalence of allergic asthma and rhinitis in children during the first year of life79 and up to 4–5 years old,80 with a more pronounced protective effect in high-risk families.
By contrast, the retrospective longitudinal case–control study PROBIT81 showed a lack of protection of breastfeeding to develop sensitisation to aeroallergens and respiratory allergy. These data have been confirmed in different meta-analyses for both asthma82 and rhinitis.83
Extended breastfeeding has been suggested as a risk factor for the development of asthma in cases of children of atopic mothers,84 but a reverse causality bias could be present in these studies, because these mothers keep voluntarily longer breastfeeding, in order to delay the contact of their children with foods, and the allergic risk observed would be more associated with familiar atopic predisposition than with the extended breastfeeding itself.85
With regard to artificial feeding, the introduction of whole milk in the first three months of life appears to increase the risk of asthma.86 Taking protein hydrolysates instead of whole milk until nine months decreased the risk of episodes of wheezing at eighteen months,87 although this protective role for asthma was lost in the subsequent years.78
Introduction of solid foods and risk of atopy: classical messages and new conceptsThe recommendations of exclusive breastfeeding in children during the first six months of life are supported in that early introduction of solid foods when the gastrointestinal mucosal barrier is immature can promote food hypersensitivity,88 although subsequent studies claim that the importance of transient immunodeficiency in the newborn has been overestimated.89
Several international guidelines were developed with the aim of minimising the risk of developing atopic diseases in children. In this sense, the European Society of Paediatric Allergy and Clinical Immunology (ESPACI) recommended postponing the introduction of solid foods until six months of age,90 the WHO proposed exclusive breastfeeding for the first six months of age and a delayed introduction of solid foods91 and the Committee on Nutrition of the AAP recommended avoidance of cow's milk until the first year of life, eggs until two years, and peanuts, tree nuts and fish until three years, especially in high-risk children.92
All these recommendations are highly controversial: they were confirmed in the meta-analysis of Fiocchi et al.,93 but they are rejected by Maloney et al.,94 who have questioned Fiocchi's conclusions because the studies included were too outdated and biased. Current evidence is insufficient to suggest that early introduction of solid foods can increase the risk of allergic asthma and rhinitis in children17,45 and therefore, its late introduction is not a consistent method for prevention of allergic diseases.95
Some authors even refer an association between a delayed introduction of foods such as wheat and an increased risk of allergy,96 but these results seem to be specifically limited to an increased sensitisation to food allergens, considering that sensitisation to aeroallergens or the occurrence of allergic rhinitis and asthma were not enhanced.
In recent years, new concepts have been established, indicating that tolerance to foods seems to be related with an early and regular exposure to food proteins during a crucial window period, at which a persistent exposure to potentially allergenic foods could promote the development of oral tolerance, rather than an increased risk of sensitisation.97 Thus, a suitable strategy of intervention at this period should reduce the incidence of allergic diseases.98 This window of confidence is not well defined, but recent studies suggest that it may be located between the fourth and seventh month of life99 and that a delayed introduction of solids beyond this period may increase food allergies, even in high-risk children.
New strategies of AAP in 2008 stated that the introduction of solid foods should be individualised for each child, depending on the level of maturity: keeping the head up, bringing the spoon to mouth, opening the mouth for food, or doubling of birth weight could indicate that it is a good time to introduce solid foods. AAP also states that exclusive breastfeeding for at least four months may be effective in decreasing the risk of atopy in children. If milk supplement is necessary, it would be preferable to use protein hydrolysates or less allergenic milk formulas. An update of AAP in 2012 states that there is no scientific evidence to confirm that the introduction of high allergy risk foods such as eggs and fish beyond the sixth month can reduce the risk of allergy.100Table 4 summarises the most recent recommendations of the AAP, the WHO and the EAACI about the most appropriate measures to reduce the allergic risk during pregnancy, lactation and the first year of life.
Feeding recommendations for the perinatal period.
| • To avoid dietary restrictions during pregnancy. |
| • Exclusive breastfeeding in the first four months, avoiding the use of whole milk. If breastfeeding is not feasible, the use of milk hydrolysates should be recommended. |
| • Introduction of solid foods within the tolerance window period (between the fourth and seventh month of life). |
But the most recent studies suggest that even these latest recommendations according to international guidelines would be questionable. A Cochrane review of Kramer et al.101 published in 2012 concluded that there was no difference in the risk of long-term development of atopic disease between exclusive breastfeeding for six months or exclusive breastfeeding for 3–4 months plus two mixed breastfeeding months thereafter. In this regard, the recently completed SEATON study102 has concluded that the duration of breastfeeding and the time of the introduction of complementary foods during infancy do not seem to have an important role in the risk to develop atopic diseases in children over the long term, not even in children at high risk for atopy.
Finally, a systematic review reported by EAACI Food Allergy and Anaphylaxis Guidelines Group in 2014103 concluded that interventions such as changing the diet or supplements of pregnant or breastfeeding women and delaying the introduction of solid foods are unlikely to be useful to protect against atopic disease.
ConclusionsFood allergy and respiratory allergy seem to be associated and this association is even more intense if food sensitisation starts early or is persistent. Furthermore, food allergy appears to be a phenotypic marker of severe atopic asthma, and on the other hand, the fact of having asthma predisposes to life-threatening reactions to foods.
The established recommendations for dietary restrictions during pregnancy and breastfeeding as a way to prevent further development of respiratory allergy are controversial and do not appear to be supported by consistent scientific data. Recent recommendations from various medical societies proposed exclusive breastfeeding during the first four months of life, with the introduction of solid foods in the period from the fourth to the seventh month.
Finally, it should be noted that there is a pronounced need for prospective longitudinal cohort studies, using strict diagnostic criteria and double-blind placebo-controlled challenge-tests combined with the assessment of respiratory function. This could avoid unjustified recommendations involving unnecessary dietary restrictions in growing children, which besides being ineffective to lower the risk for the subsequent development of allergic disorders, may induce severe nutritional defects.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors have no conflict of interest to declare.