Research on potential risk factors of asthma can enhance our understanding of geographic differences and inform decisions on preventive strategies.
MethodsIn 2002, a cross-sectional population-based study was carried out in the area of Castellon (Spain), following the International Study of Asthma and Allergies in Childhood (ISAAC) Phase III methodology. Asthma symptoms and related risk factor questionnaires were completed by parents of 6-7 year-old schoolchildren. Logistic regression was used in the analysis.
ResultsParticipation rate was 88 % (4492 of 4872 schoolchildren). Prevalence of wheeze in the past year, asthma ever, and physician-diagnosed asthma were 8 %, 7 % and 6 %, respectively. Risk factors independently associated with all three asthma case definitions were history of bronchitis or pneumonia, allergic rhinitis, family members with atopic disease, and residing in an industrialised area. Risk factors for asthma ever and physician-diagnosed asthma were male sex, atopic eczema and presence of a dog at home; exclusive breast-feeding and the presence of another animal (not a dog or cat) were protective factors. Maternal age was inversely related to physician-diagnosed asthma. Residence in an area of heavy truck traffic and the father smoking at home were associated with asthma ever. Risk factors for wheeze in the past year were low social class, history of sinusitis and the father smoking at home.
ConclusionsEnvironmental factors are related to the presence of asthma. Preventive measures should be directed to improving air pollution, promoting breast-feeding and reducing smoking in the home.
Asthma is an inflammatory respiratory disease following a chronic course, with an unclear etiology and marked geographic variations in its prevalence. Asthma symptoms in 6–7year-old schoolchildren vary from 5 % in Albania to 37 % in Costa Rica1. While Spain has an intermedíate position regarding asthma prevalence, important differences among Spanish areas have been described2 and an increasing prevalence in 6–7year-old Spanish schoolchildren was observed between 1994 and 20023.
In Castellon, the prevalence of asthma is low when compared to other Spanish areas2,4 or other European countries1. Nevertheless, an increase in asthma was observed in 6–7year-old schoolchildren between Phases I and III of the ISAAC study in Castellon2. Research on potential risk factors for asthma and knowledge on the impact of these factors in a low prevalence area can contribute to a better understanding of geographic differences and inform the implementation of preventive strategies.
Materials and MethodsIn 2002, a cross-sectional population based study, following the ISAAC Phase III methodology5, was carried out in the area of Castellon (Spain). This area, situated in the east of Spain on the Mediterranean coast, has a population of 267,000 and covers 388.7 km2. First year primary school children of 6–7years old were included as previously described6. The Asturias Ethics Committee (Spain) approved Phase III of the ISAAC study for all Spanish centres. After informed consent, the core ISAAC study questionnaire and an additional risk factor questionnaire were completed by the parents of the schoolchildren.
Three mutually non-exclusive asthma case definitions were defined by an affirmative answer to any one of the following questions: a) “Has your child had wheezing in the last 12months?” b) “Has your child ever had asthma?” and c) “Has a physician ever diagnosed your child as suffering from asthma?” All children with an affirmative answer to question “a” were included in the “wheeze in past year” group; all children with an affirmative answer to question “b” in the “asthma ever” group; and all those with an affirmative answer to question “c” in the “physician-diagnosed asthma” group.
The study of risk factors comprised the following aspects: age; sex; residence; parents' social class7; parents' age; country of birth; duration of residence; history of allergic disease (asthma, allergic rhinitis and atopic dermatitis), both personal and in other family members; maternal age; number of siblings; exclusive breastfeeding; personal history of otitis, sinusitis, pneumonia, bronchitis; attending day care; exposure to smoke at home (mother, father and others smoking at home); presence of pets at home (dog, cat, and others); frequency of truck traffic in the street of residence; and service or industrial zone of residence. The industrial zone comprised the urban conurbation of Vila-real, L'Alcora, Almassora and El Grau with its ceramic tile and petrochemical industries.
Statistical methodsThe prevalence of each asthma case definition was estimated by dividing the number of cases by the number of schoolchildren included. The odds ratios (OR) and their 95 % confidence intervals (CI) were calculated for each risk factor with respect to each case definition; an approximate chi-squared test of homogeneity of odds and a test for linear trend of the log odds against the numerical code used for the categories of risk factors were estimated. Both of these tests were based on the score statistic and its variance. Logistic regression models were used to adjust for potential confounding variables. Logistic models included age and significant variables (p < 0.05). All model goodness of fit (p > 0.05) was estimated by the Hosmer-Lemeshow test. All analyses were performed with STATA, version 9.0.
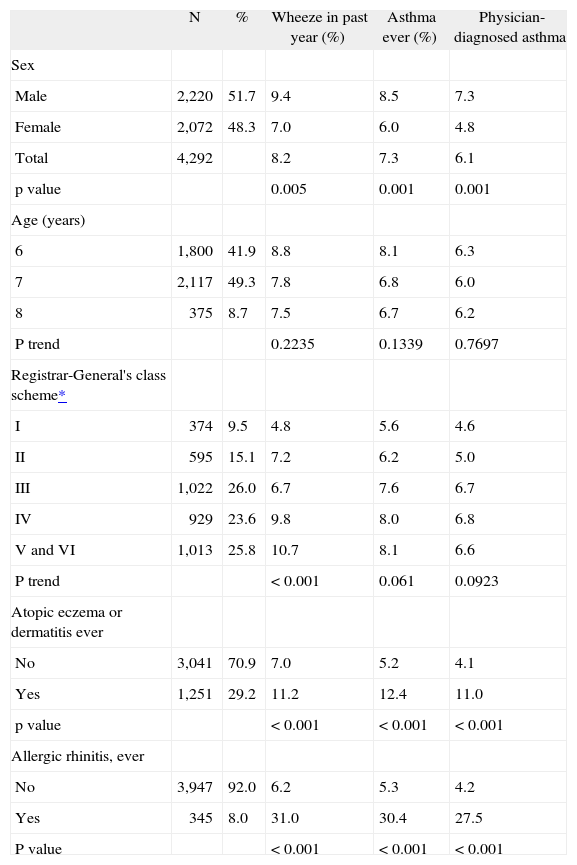
ResultsAll 63 schools in the area of Castellon agreed to participate in the study. Parents of children attending the first year of primary education (ages 6–7years) were contacted to take part in the survey, and the parents of 4292 (88.1 %) out of 4872 children consented to participate in the study; 375 (8.7 %) children were eight years old when the questionnaire was completed. Wheeze in the past year was reported by 8 %, asthma ever by 7 % and physician-diagnosed asthma by 6 % (Table I).
Distribution of asthma case definitions by sex, age, socio-economic level and history of dermatitis or rhinitis
| N | % | Wheeze in past year (%) | Asthma ever (%) | Physician-diagnosed asthma | |
| Sex | |||||
| Male | 2,220 | 51.7 | 9.4 | 8.5 | 7.3 |
| Female | 2,072 | 48.3 | 7.0 | 6.0 | 4.8 |
| Total | 4,292 | 8.2 | 7.3 | 6.1 | |
| p value | 0.005 | 0.001 | 0.001 | ||
| Age (years) | |||||
| 6 | 1,800 | 41.9 | 8.8 | 8.1 | 6.3 |
| 7 | 2,117 | 49.3 | 7.8 | 6.8 | 6.0 |
| 8 | 375 | 8.7 | 7.5 | 6.7 | 6.2 |
| P trend | 0.2235 | 0.1339 | 0.7697 | ||
| Registrar-General's class scheme* | |||||
| I | 374 | 9.5 | 4.8 | 5.6 | 4.6 |
| II | 595 | 15.1 | 7.2 | 6.2 | 5.0 |
| III | 1,022 | 26.0 | 6.7 | 7.6 | 6.7 |
| IV | 929 | 23.6 | 9.8 | 8.0 | 6.8 |
| V and VI | 1,013 | 25.8 | 10.7 | 8.1 | 6.6 |
| P trend | < 0.001 | 0.061 | 0.0923 | ||
| Atopic eczema or dermatitis ever | |||||
| No | 3,041 | 70.9 | 7.0 | 5.2 | 4.1 |
| Yes | 1,251 | 29.2 | 11.2 | 12.4 | 11.0 |
| p value | < 0.001 | < 0.001 | < 0.001 | ||
| Allergic rhinitis, ever | |||||
| No | 3,947 | 92.0 | 6.2 | 5.3 | 4.2 |
| Yes | 345 | 8.0 | 31.0 | 30.4 | 27.5 |
| P value | < 0.001 | < 0.001 | < 0.001 |
Significant differences in prevalence were observed by sex for all three asthma case definitions: all were less frequent in females than in males (Table I). A significant trend was observed between social class and wheeze in the past year, with a prevalence of 5 % in class I (higher) versus 11 % in class V-VI (lower). History of atopic disease (eczema or allergic rhinitis) was strongly associated with any one of the asthma case definitions; this association was especially manifest with allergic rhinitis, in the presence of which the prevalence of asthma case definitions was 30 % as opposed to 6 % to 4 % in its absence (Table I).
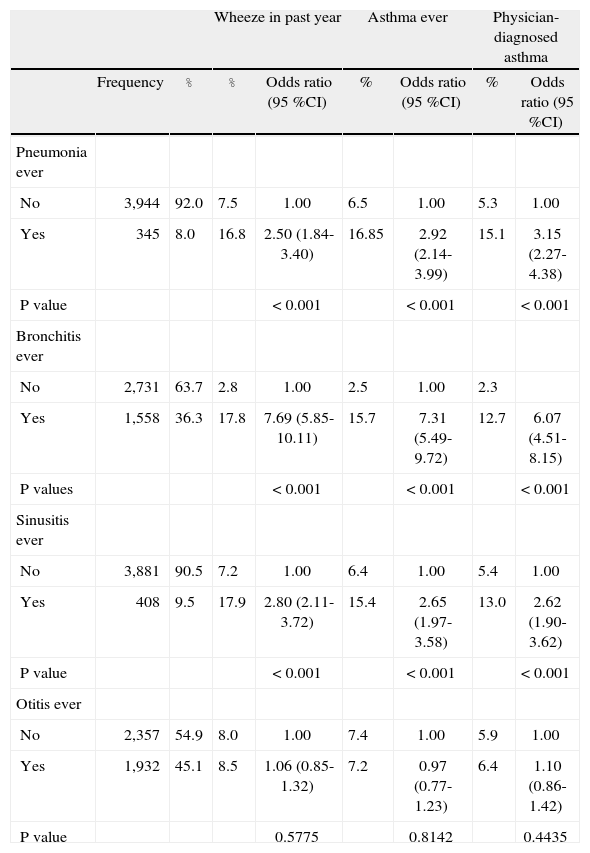
Some manifestations of respiratory infectious disease were significantly more frequent in children with any one of the asthma case definitions (Table II). Between 13 to 18 % of children with previous episodes of pneumonia, bronchitis or sinusitis also referred to wheeze episodes in the past year, asthma ever or asthma diagnosed by a physician, compared to 2 to 7 % of those who did not refer to any of those episodes. This association was especially strong for previous bronchitis episodes, in which case the odds of any one of the asthma related case definitions were 6 to 7 times those of children without previous bronchitis episodes (Table II).
Distribution and effects of physician-diagnosed respiratory infectious disease on asthma case definitions
| Wheeze in past year | Asthma ever | Physician-diagnosed asthma | ||||||
| Frequency | % | % | Odds ratio (95 %CI) | % | Odds ratio (95 %CI) | % | Odds ratio (95 %CI) | |
| Pneumonia ever | ||||||||
| No | 3,944 | 92.0 | 7.5 | 1.00 | 6.5 | 1.00 | 5.3 | 1.00 |
| Yes | 345 | 8.0 | 16.8 | 2.50 (1.84-3.40) | 16.85 | 2.92 (2.14-3.99) | 15.1 | 3.15 (2.27-4.38) |
| P value | < 0.001 | < 0.001 | < 0.001 | |||||
| Bronchitis ever | ||||||||
| No | 2,731 | 63.7 | 2.8 | 1.00 | 2.5 | 1.00 | 2.3 | |
| Yes | 1,558 | 36.3 | 17.8 | 7.69 (5.85-10.11) | 15.7 | 7.31 (5.49-9.72) | 12.7 | 6.07 (4.51-8.15) |
| P values | < 0.001 | < 0.001 | < 0.001 | |||||
| Sinusitis ever | ||||||||
| No | 3,881 | 90.5 | 7.2 | 1.00 | 6.4 | 1.00 | 5.4 | 1.00 |
| Yes | 408 | 9.5 | 17.9 | 2.80 (2.11-3.72) | 15.4 | 2.65 (1.97-3.58) | 13.0 | 2.62 (1.90-3.62) |
| P value | < 0.001 | < 0.001 | < 0.001 | |||||
| Otitis ever | ||||||||
| No | 2,357 | 54.9 | 8.0 | 1.00 | 7.4 | 1.00 | 5.9 | 1.00 |
| Yes | 1,932 | 45.1 | 8.5 | 1.06 (0.85-1.32) | 7.2 | 0.97 (0.77-1.23) | 6.4 | 1.10 (0.86-1.42) |
| P value | 0.5775 | 0.8142 | 0.4435 | |||||
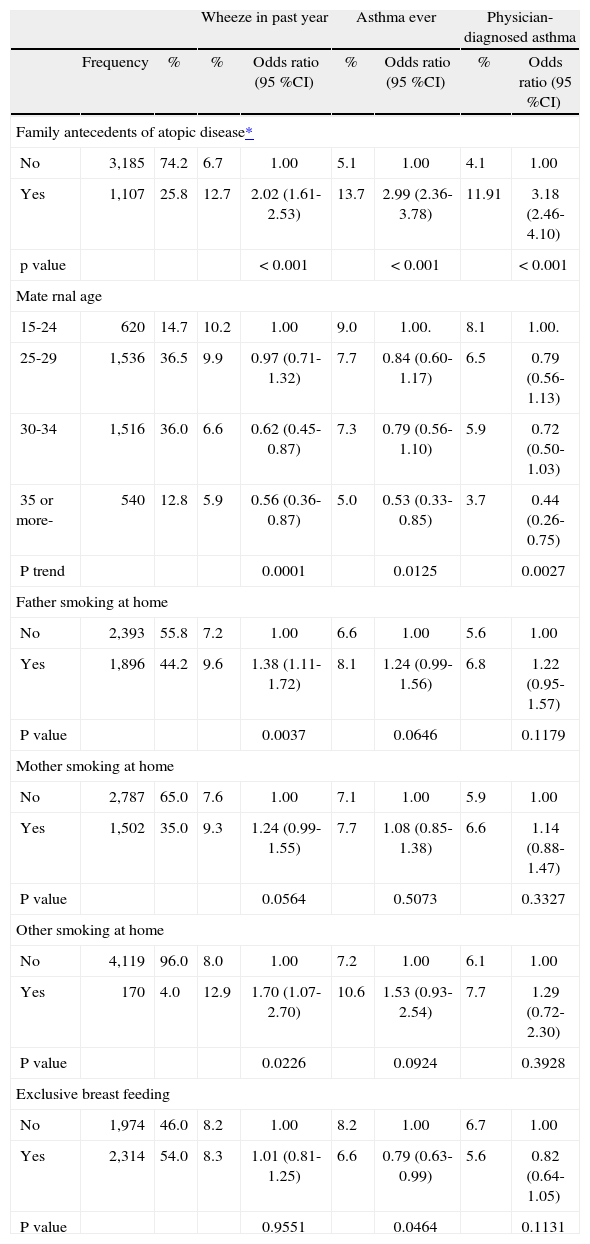
A history of atopic disease in other family members was reported in 26 % of the children included in the study (Table III). Those children suffered a high risk of wheeze in the past year, asthma ever or physician-diagnosed asthma, compared to those with no family history of atopic disease (Table III). Maternal age showed a significant inverse trend with the prevalence of asthma, with a higher frequency of all case definitions in children of younger mothers. Wheeze in the past year was significantly more common for children whose father or another household member smokes at home; this association was less manifest when the mother reportedly smokes at home. There was no association with smokers in the family and asthma ever or physician-diagnosed asthma. History of exclusive breastfeeding was protective for asthma ever and physician-diagnosed asthma.
Distribution and effects of family associated factors and exclusive breast-feeding on asthma case definitions
| Wheeze in past year | Asthma ever | Physician-diagnosed asthma | ||||||
| Frequency | % | % | Odds ratio (95 %CI) | % | Odds ratio (95 %CI) | % | Odds ratio (95 %CI) | |
| Family antecedents of atopic disease* | ||||||||
| No | 3,185 | 74.2 | 6.7 | 1.00 | 5.1 | 1.00 | 4.1 | 1.00 |
| Yes | 1,107 | 25.8 | 12.7 | 2.02 (1.61-2.53) | 13.7 | 2.99 (2.36-3.78) | 11.91 | 3.18 (2.46-4.10) |
| p value | < 0.001 | < 0.001 | < 0.001 | |||||
| Mate rnal age | ||||||||
| 15-24 | 620 | 14.7 | 10.2 | 1.00 | 9.0 | 1.00. | 8.1 | 1.00. |
| 25-29 | 1,536 | 36.5 | 9.9 | 0.97 (0.71-1.32) | 7.7 | 0.84 (0.60-1.17) | 6.5 | 0.79 (0.56-1.13) |
| 30-34 | 1,516 | 36.0 | 6.6 | 0.62 (0.45-0.87) | 7.3 | 0.79 (0.56-1.10) | 5.9 | 0.72 (0.50-1.03) |
| 35 or more- | 540 | 12.8 | 5.9 | 0.56 (0.36-0.87) | 5.0 | 0.53 (0.33-0.85) | 3.7 | 0.44 (0.26-0.75) |
| P trend | 0.0001 | 0.0125 | 0.0027 | |||||
| Father smoking at home | ||||||||
| No | 2,393 | 55.8 | 7.2 | 1.00 | 6.6 | 1.00 | 5.6 | 1.00 |
| Yes | 1,896 | 44.2 | 9.6 | 1.38 (1.11-1.72) | 8.1 | 1.24 (0.99-1.56) | 6.8 | 1.22 (0.95-1.57) |
| P value | 0.0037 | 0.0646 | 0.1179 | |||||
| Mother smoking at home | ||||||||
| No | 2,787 | 65.0 | 7.6 | 1.00 | 7.1 | 1.00 | 5.9 | 1.00 |
| Yes | 1,502 | 35.0 | 9.3 | 1.24 (0.99-1.55) | 7.7 | 1.08 (0.85-1.38) | 6.6 | 1.14 (0.88-1.47) |
| P value | 0.0564 | 0.5073 | 0.3327 | |||||
| Other smoking at home | ||||||||
| No | 4,119 | 96.0 | 8.0 | 1.00 | 7.2 | 1.00 | 6.1 | 1.00 |
| Yes | 170 | 4.0 | 12.9 | 1.70 (1.07-2.70) | 10.6 | 1.53 (0.93-2.54) | 7.7 | 1.29 (0.72-2.30) |
| P value | 0.0226 | 0.0924 | 0.3928 | |||||
| Exclusive breast feeding | ||||||||
| No | 1,974 | 46.0 | 8.2 | 1.00 | 8.2 | 1.00 | 6.7 | 1.00 |
| Yes | 2,314 | 54.0 | 8.3 | 1.01 (0.81-1.25) | 6.6 | 0.79 (0.63-0.99) | 5.6 | 0.82 (0.64-1.05) |
| P value | 0.9551 | 0.0464 | 0.1131 | |||||
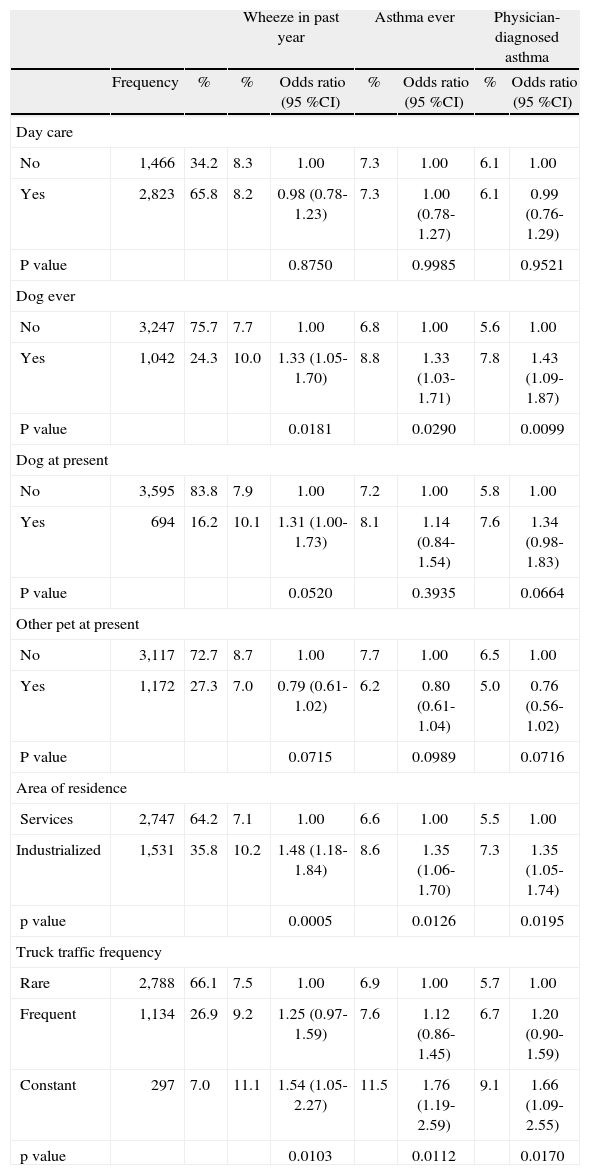
No relationship was observed with day care attendance (Table IV). The presence of pets at home was only associated with a greater risk of asthma where a dog had ever been kept as a pet; no association was identified for cats or other animals (Table IV). Finally, area of residence was significantly associated with all three asthma case definitions described, as was the intensity of truck traffic near the home, with a significant association for trend (Table IV).
Distribution and effects of day care attendance and environmental factors on asthma case definitions
| Wheeze in past year | Asthma ever | Physician-diagnosed asthma | ||||||
| Frequency | % | % | Odds ratio (95 %CI) | % | Odds ratio (95 %CI) | % | Odds ratio (95 %CI) | |
| Day care | ||||||||
| No | 1,466 | 34.2 | 8.3 | 1.00 | 7.3 | 1.00 | 6.1 | 1.00 |
| Yes | 2,823 | 65.8 | 8.2 | 0.98 (0.78-1.23) | 7.3 | 1.00 (0.78-1.27) | 6.1 | 0.99 (0.76-1.29) |
| P value | 0.8750 | 0.9985 | 0.9521 | |||||
| Dog ever | ||||||||
| No | 3,247 | 75.7 | 7.7 | 1.00 | 6.8 | 1.00 | 5.6 | 1.00 |
| Yes | 1,042 | 24.3 | 10.0 | 1.33 (1.05-1.70) | 8.8 | 1.33 (1.03-1.71) | 7.8 | 1.43 (1.09-1.87) |
| P value | 0.0181 | 0.0290 | 0.0099 | |||||
| Dog at present | ||||||||
| No | 3,595 | 83.8 | 7.9 | 1.00 | 7.2 | 1.00 | 5.8 | 1.00 |
| Yes | 694 | 16.2 | 10.1 | 1.31 (1.00-1.73) | 8.1 | 1.14 (0.84-1.54) | 7.6 | 1.34 (0.98-1.83) |
| P value | 0.0520 | 0.3935 | 0.0664 | |||||
| Other pet at present | ||||||||
| No | 3,117 | 72.7 | 8.7 | 1.00 | 7.7 | 1.00 | 6.5 | 1.00 |
| Yes | 1,172 | 27.3 | 7.0 | 0.79 (0.61-1.02) | 6.2 | 0.80 (0.61-1.04) | 5.0 | 0.76 (0.56-1.02) |
| P value | 0.0715 | 0.0989 | 0.0716 | |||||
| Area of residence | ||||||||
| Services | 2,747 | 64.2 | 7.1 | 1.00 | 6.6 | 1.00 | 5.5 | 1.00 |
| Industrialized | 1,531 | 35.8 | 10.2 | 1.48 (1.18-1.84) | 8.6 | 1.35 (1.06-1.70) | 7.3 | 1.35 (1.05-1.74) |
| p value | 0.0005 | 0.0126 | 0.0195 | |||||
| Truck traffic frequency | ||||||||
| Rare | 2,788 | 66.1 | 7.5 | 1.00 | 6.9 | 1.00 | 5.7 | 1.00 |
| Frequent | 1,134 | 26.9 | 9.2 | 1.25 (0.97-1.59) | 7.6 | 1.12 (0.86-1.45) | 6.7 | 1.20 (0.90-1.59) |
| Constant | 297 | 7.0 | 11.1 | 1.54 (1.05-2.27) | 11.5 | 1.76 (1.19-2.59) | 9.1 | 1.66 (1.09-2.55) |
| p value | 0.0103 | 0.0112 | 0.0170 | |||||
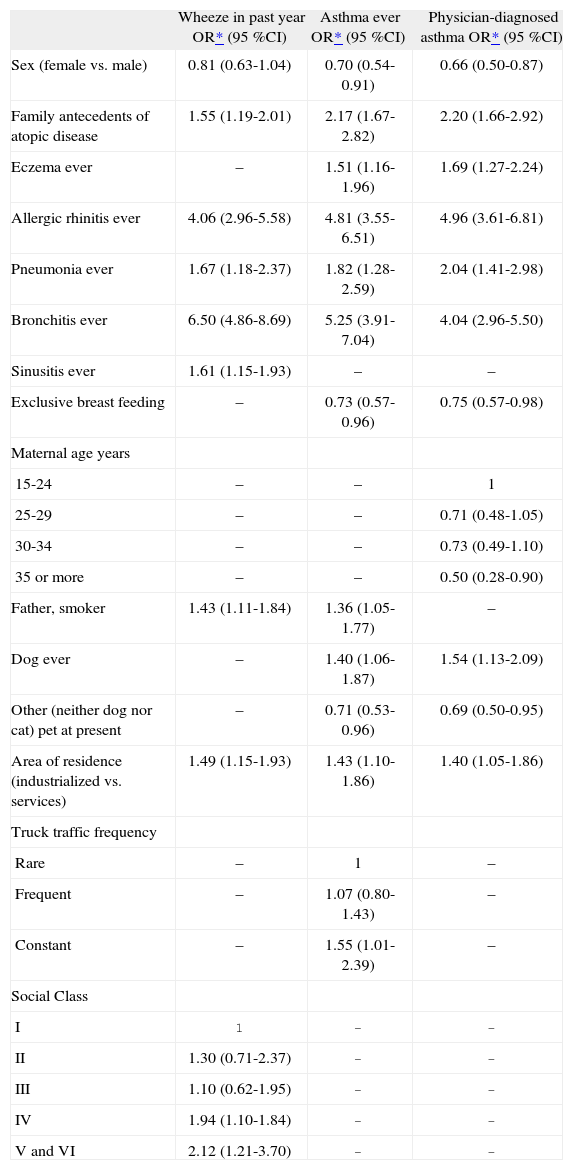
Personal antecedents of allergic rhinitis, pneumonia, or bronchitis, family history of atopic disease, and industrialised area of residence were independently associated with all three asthma case definitions. Being female constituted a protective factor for all three case definitions, although it was not significant for wheeze in the past year (Table V).
Multivariate effects of various risk factors upon asthma related case definitions
| Wheeze in past year OR* (95 %CI) | Asthma ever OR* (95 %CI) | Physician-diagnosed asthma OR* (95 %CI) | |
| Sex (female vs. male) | 0.81 (0.63-1.04) | 0.70 (0.54-0.91) | 0.66 (0.50-0.87) |
| Family antecedents of atopic disease | 1.55 (1.19-2.01) | 2.17 (1.67-2.82) | 2.20 (1.66-2.92) |
| Eczema ever | – | 1.51 (1.16-1.96) | 1.69 (1.27-2.24) |
| Allergic rhinitis ever | 4.06 (2.96-5.58) | 4.81 (3.55-6.51) | 4.96 (3.61-6.81) |
| Pneumonia ever | 1.67 (1.18-2.37) | 1.82 (1.28-2.59) | 2.04 (1.41-2.98) |
| Bronchitis ever | 6.50 (4.86-8.69) | 5.25 (3.91-7.04) | 4.04 (2.96-5.50) |
| Sinusitis ever | 1.61 (1.15-1.93) | – | – |
| Exclusive breast feeding | – | 0.73 (0.57-0.96) | 0.75 (0.57-0.98) |
| Maternal age years | |||
| 15-24 | – | – | 1 |
| 25-29 | – | – | 0.71 (0.48-1.05) |
| 30-34 | – | – | 0.73 (0.49-1.10) |
| 35 or more | – | – | 0.50 (0.28-0.90) |
| Father, smoker | 1.43 (1.11-1.84) | 1.36 (1.05-1.77) | – |
| Dog ever | – | 1.40 (1.06-1.87) | 1.54 (1.13-2.09) |
| Other (neither dog nor cat) pet at present | – | 0.71 (0.53-0.96) | 0.69 (0.50-0.95) |
| Area of residence (industrialized vs. services) | 1.49 (1.15-1.93) | 1.43 (1.10-1.86) | 1.40 (1.05-1.86) |
| Truck traffic frequency | |||
| Rare | – | 1 | – |
| Frequent | – | 1.07 (0.80-1.43) | – |
| Constant | – | 1.55 (1.01-2.39) | – |
| Social Class | |||
| I | 1 | – | – |
| II | 1.30 (0.71-2.37) | – | – |
| III | 1.10 (0.62-1.95) | – | – |
| IV | 1.94 (1.10-1.84) | – | – |
| V and VI | 2.12 (1.21-3.70) | – | – |
–: Not included in the model.
Asthma ever and physician-diagnosed asthma were independently associated with eczema and ever having had a dog, while the current presence of another pet (not a dog or a cat) or antecedents of exclusive breast feeding acted as protective factors for asthma ever and physician-diagnosed asthma (Table V).
Maternal age was inversely associated with a greater risk of physician-diagnosed asthma. Intensity of truck traffic was independently associated with asthma ever (Table V). Finally, social class showed a clear trend association with wheeze episodes in the past year, with a higher risk in those of social class category IV or V and VI. The presence of a father smoking at home and antecedents of sinusitis were also independently associated with wheeze episodes in the past year (Table V).
DiscussionOur results indicate that a family history of atopic disease, personal antecedents of allergic rhinitis, low respiratory infectious diseases (pneumonia or bronchitis) and residing in an industrial area are associated with wheeze in the past year, asthma ever and physician-diagnosed asthma, thus lending support to a multifactor origin of asthma.
While the contribution of inherited factors is well accepted, the contribution of respiratory infectious disease and industrial area of residence are controversial8,9. Some cohort studies have identified low infectious respiratory disease and, more specifically, infections by respiratory syncytial virus or rhinovirus as risk factors for future asthma10,11. Despite the fact that the role of lower respiratory infections in the etiology or exacerbations of asthma remains controversial, in our study antecedents of bronchitis showed a strong association with all the three case definitions in agreement with the study of incidence of asthma in Castellon4, and other studies12,13.
The residential area can be considered as a proxy for air pollution exposure. Air pollution is considered to be associated with respiratory infections, asthma and atopic disease14–16. Our study shows that residing in heavily industrialised areas, compared to service areas, has a constant effect on the presence of all asthma case definitions analysed, suggesting that it could act synergistically with other risk factors. Similar results were obtained when the duration of residence in the area was considered.
The prevalence of wheeze in the past year was associated with the risk factors mentioned above and with a history of sinusitis, the father smoking at home, and social class. Social class had a significant response gradient with more asthma symptoms in lower social class levels. These associations have been observed in other studies and in the Phase I ISAAC Castellon study17–21, and could be explained by high susceptibility and exposure to risk factors of asthma in low social class: inferior environmental and living conditions with increasing outdoor and indoor air pollution, less healthy behaviour, such as more smoking prevalence, more psychosocial stress and less access to quality medical care22. Low social class and respiratory infections were associated with current wheezing against the “hygiene hypothesis#x201D; such as it has been indicated in developing America Latin countries with high prevalence of asthma and high frequent of acute respiratory infections23. Alternative hypothesis of asthma time trends include changes in air pollution, maternal factors, diet and obesity, and viral respiratory infections24.
Risk factors for asthma ever and physician-diagnosed asthma were similar. Male sex, family history of atopic disease, history of atopic eczema, history of allergic rhinitis, pneumonia, bronchitis, area of residence, and presence of dog at home were associated with these two case definitions. Male sex as a risk factor and history of atopic eczema in children are well established25. The presence of a dog at home that could modulate the atopic response is described in some studies as a protective factor and in others as a risk factor26,27. The observed effect of breast-feeding is consistent with previous observations28, although in a recent intervention study no effect was observed29.
For asthma ever, father smoking at home and frequency of truck traffic support the importance of environmental factors in the development of the disease30. For physician-diagnosed asthma, higher maternal age was associated with less risk of disease, as has been observed by others31,32, but no association with asthma ever or physician-diagnosed asthma was identified when we measured the effect of the number of siblings.
The strengths of our study lie in the high participation rate, the use of a validated core questionnaire33, the control for potential confusion factors by logistic regression, and the inclusion of information on such factors as genetic background, number of siblings, maternal age, breast feeding, day care attendance, environmental exposures, characteristics of the area of residence and duration of residence.
The study limitations include the subjectivity of the measure of disease, related to the accuracy of the parents reporting wheeze and the diagnosis of asthma34, the cross-sectional design35, and that information on risk factors such as diet or home characteristics was not collected. Recall bias cannot be dismissed, since parents of asthmatic children could report more frequent respiratory infections or family histories of atopic disease. Differences on reporting smoking at home by parents in relation to the health status of their children cannot be excluded. Bias by reverse causation can be present in the apparent protection effect of the presence of pets other than a dog or a cat. Finally, as the children's age, 6–7years old, is considered to be the final chronological stage of the phenotype of transitory wheezing or non-atopic asthma and the beginning of atopic asthma36, the risk factors observed could be a mix of genetic predisposition and environmental exposure.
Our study was conducted in an area with a very low prevalence of asthma. The risk factors most frequently associated with any one of the three case definitions were social class IV-VI (48 %), father smoking at home (44 %), history of bronchitis (36 %), residence in an industrial area (36 %) and frequent constant truck traffic (34 %), suggesting the importance of environmental factors in the genesis and development of the disease in the children included in the study. When comparisons have been made with countries with low prevalence of asthma, such as Greece and Albania in the South of Europe, risk factors of the disease were parental history of asthma, exposure of air pollution, severe respiratory infections, exposure to passive smoking, and low socioeconomic level. In addition, Mediterranean diet, breast-feeding, attending day care and shared room were protectors against the disease37–39. On the other hand, climatic conditions play a role in the low prevalence of asthma40.
Preventive measures aimed at improving air pollution and economic conditions, the promotion of exclusive breast-feeding and the reduction of smoking at home are clearly justified in order to diminish the prevalence of asthma.
The authors thank the schoolchildren, their parents, teachers and heads of schools for their participation in the study, and the Education Department for its support.