Atopy is an important risk factor for asthma, rhinitis, atopic eczema and urticaria. For this reason, several studies have been done to determine the prevalence of atopy in the paediatric population. The important differences among these studies do not allow the extrapolating of results. In this study, we calculate the prevalence of atopy and atopy-related diseases in a paediatric population using a different methodology.
MethodsRetrospective study among children referred for drug allergy in which the latter was discarded. We evaluated the prevalence of atopy (measured by allergen sensitisation), asthma, rhinitis, urticaria, atopic eczema and their characteristics.
ResultsThree hundred and forty-two patients were studied for adverse drug reaction. This was discarded in 325/342 patients. 20 % of the children in the sample were atopic. Atopy prevalence increased with age. Some atopy related disease was observed in 83/325 (25.5 %) children. Among these children allergen sensitisation increased from 42.3 % in the 0-3 years age group to 93.3 % in the 7-14 age group (p < 0.0001). Prevalence of asthma was 11.5 %, 10.2 % and 7 % in the 0-3, 4-6 and 7-14 age groups, respectively. Prevalence of rhinoconjunctivitis increased through age groups with a prevalence of 20 % among the 7 to 14-year old children.
ConclusionThe use of this type of methodology seems to be correct to estimate the prevalence of atopy. Prevalence of allergen sensitisation is very high among 7 to 14-year old children with asthma and/or rhinoconjunctivitis.
Atopy can be defined as a phenotype with an increased risk of IgE-mediated diseases as its main characteristic. Its aetiology has probably a genetic predisposition as suggested by studies where the prevalence of atopy among siblings of atopic individuals was six times higher.1 However, genetic predisposition is not the only factor involved. Epidemiological studies show a higher prevalence in metropolitan areas and western countries compared to rural areas and non-industrialised countries.2–4 These data suggest that environmental factors are important contributors to the prevalence of atopy.
Atopy has been strongly related to asthma, rhinitis and allergic eczema. For this reason, in the last few years, there has been an increasing interest to determine the real prevalence of atopy and its impact on atopy related diseases. Multiple studies in several parts of the world, including a multinational collaborative initiative, the ISAAC study, have been carried out with this purpose. Studies have usually been based on questionnaires and skin prick tests (SPT) performed amongst school pupils. Unfortunately, the very high variability of the results (20 to 60-fold difference) has caused a lot of confusion. Differences reported even in close geographic regions do not permit estimations of the prevalence of atopy, asthma or rhinitis in other areas3. Moreover, there are also data that suggest an increase in the prevalence of atopy.6–8 As a result of the different prevalence of atopy reported, the question about which factors are involved in these differences has been raised. Factors such as migration flows, environmental or genetic changes, and methodological biases could contribute to these differences, but further investigation is needed.
In this paper we evaluate a different strategy to determine the prevalence of atopy and atopy-related diseases. A sample of children referred to our allergy department for evaluation of possible allergic reactions to drugs were retrospectively studied to determine the prevalence of atopy and atopy related diseases in our children population.
Material and methodsStudy populationThis study was conducted at Elche General University Hospital, a tertiary care hospital in the city of Elche (Alicante, Spain), with a catchment area of 330,000 inhabitants and 57,000 children less than 15years of age. The city is situated on the eastern coast of Spain, 10km from the sea. The Allergy Department of our hospital is the only paediatric allergy department in the city and its surroundings. All allergic diseases and non-allergic asthma in children are referred to this hospital.
Requests of studies for drug allergy account for 9 % of the appointments in our department. Following SPT; intradermal tests; IgE specific tests; and provocation tests, only 9 % of the patients had a drug allergy confirmed. The remaining 91 % of the children studied were considered representative of the general population.
Study designA retrospective review of the clinical records of the children attended from 2000 to 2007 for an allergic drug reaction was performed. Only patients whose main appointment request was a suspicion of drug allergy were included for this study. Data on asthma or asthma symptoms, rhinoconjunctivitis, allergic eczema, urticaria, other allergic symptoms, allergen sensitivity in SPT, age, sex and family history of atopy were analysed.
All the children attended for the first time at our allergy office had a thorough anamnesis (which always included rhinitis, asthma, urticaria, allergic symptoms, and family atopic symptoms or diseases); were systematically tested with SPT; and had pulmonary function tests if asthma symptoms were reported.
Skin prick testPatients were tested with a panel of the most common food and inhaled allergens (ALK-Abello): Dermatophagoides pteronyssinus, Dermatophagoides farinae, Blatella germanica, cat dander, dog dander, rabbit dander, horse dander, Alternaria, Arpergillus, Olea europaea, Artemisia, Parietaria judaica, mixed grasses (Dactiylis, Lolium, Festuca, Poa, Phelum and Avena), cow's milk, egg white and blue fish, almond, peanut, orange and peach. Histamine (10mg/ml) and saline solution (0.9 %) were used as positive and negative controls, respectively. SPT were performed by an expert nurse with ALK-Abello (Madrid, Spain) lancets. A child was judged to be sensitised to a specific allergen if a wheal diameter measuring 3mm or more was observed 15 minutes after the prick.
Diagnostic definitionsAtopy was defined as a sensitisation to one or more allergens measured by SPT. The atopy related diseases (ARD) considered were: asthma, rhinoconjunctivitis, atopic dermatitis and urticaria. Current asthma was defined as a department doctor's diagnosis. Rhinoconjunctivitis diagnosis required frequent or seasonal symptoms with two or more of the following: itchy nose; rhinorrhoea; sneezing; sneezing fit; nasal blockage; red and itchy eyes. Atopic dermatitis (AD) was diagnosed following the Hanifin and Rajka diagnostic criteria.9 If the child had an absence of symptoms during the past 12months it was considered that he had overgrown AD. Urticaria was diagnosed for the study if the patient had gone through repetitive urticaria lesions in the last 12months. Family history of atopy was defined as one or more of the following: physician diagnosed asthma; suggestive history of allergic rhinitis/conjunctivitis; physician diagnosed allergic eczema; or any other confirmed IgE mediated allergies in at least one parent or sibling.
Statistical analysisMaximal sample size was estimated on 321 patients. Sample size was calculated for prevalence of atopy and atopy related diseases with estimated proportions of 3–30 %, precision 1–5 % and 95 % confidence level. For comparisons, children were distributed following the Tucson Cohort10 into 3 age groups: 0 to 3years, 4 to 6years and 7 to 14years of age. x2-test was used to compare prevalence between groups. Statistical calculations were done using EPIDAT version 3.0 software package (WHO) and statistical significance was set at p < 0.05.
ResultsGeneral characteristicsFrom 2000 to 2007, 342 patients were attended in our paediatric allergy department with the clinical suspicion of drug allergy. Of them, adverse drug reaction was discarded in 325 patients and they were used as the study sample. 162/325 (49.8 %; IC95 %: 44.3-55.4) were male. Sex distribution was homogeneous among the three age groups (50 ± 2 %). A statistically significant difference (p < 0.005) was observed in sex distribution in atopic children; 43/65 (66 %) were boys. This male predominance was especially marked in atopics in the 4-6-year age group (29 % male vs. 71 % female). The mean age of the patients was 5.6years (range, 0–14years).
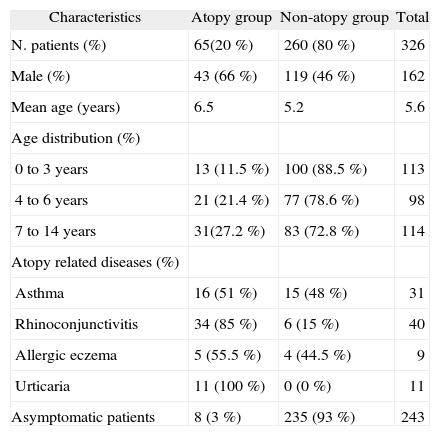
Prevalence of atopyAtopy was diagnosed by skin prick test in 65/325 (20 %; IC95 %: 15.5-24.5 %) patients. Atopy increased with age with a 16 % difference between the 0-3-year and 7-14-year age groups (p < 0.005). Differences between the atopic and non-atopic are summarized in Table I.
Main differences between the atopy and non-atopy groups
| Characteristics | Atopy group | Non-atopy group | Total |
| N. patients (%) | 65(20 %) | 260 (80 %) | 326 |
| Male (%) | 43 (66 %) | 119 (46 %) | 162 |
| Mean age (years) | 6.5 | 5.2 | 5.6 |
| Age distribution (%) | |||
| 0 to 3years | 13 (11.5 %) | 100 (88.5 %) | 113 |
| 4 to 6years | 21 (21.4 %) | 77 (78.6 %) | 98 |
| 7 to 14years | 31(27.2 %) | 83 (72.8 %) | 114 |
| Atopy related diseases (%) | |||
| Asthma | 16 (51 %) | 15 (48 %) | 31 |
| Rhinoconjunctivitis | 34 (85 %) | 6 (15 %) | 40 |
| Allergic eczema | 5 (55.5 %) | 4 (44.5 %) | 9 |
| Urticaria | 11 (100 %) | 0 (0 %) | 11 |
| Asymptomatic patients | 8 (3 %) | 235 (93 %) | 243 |
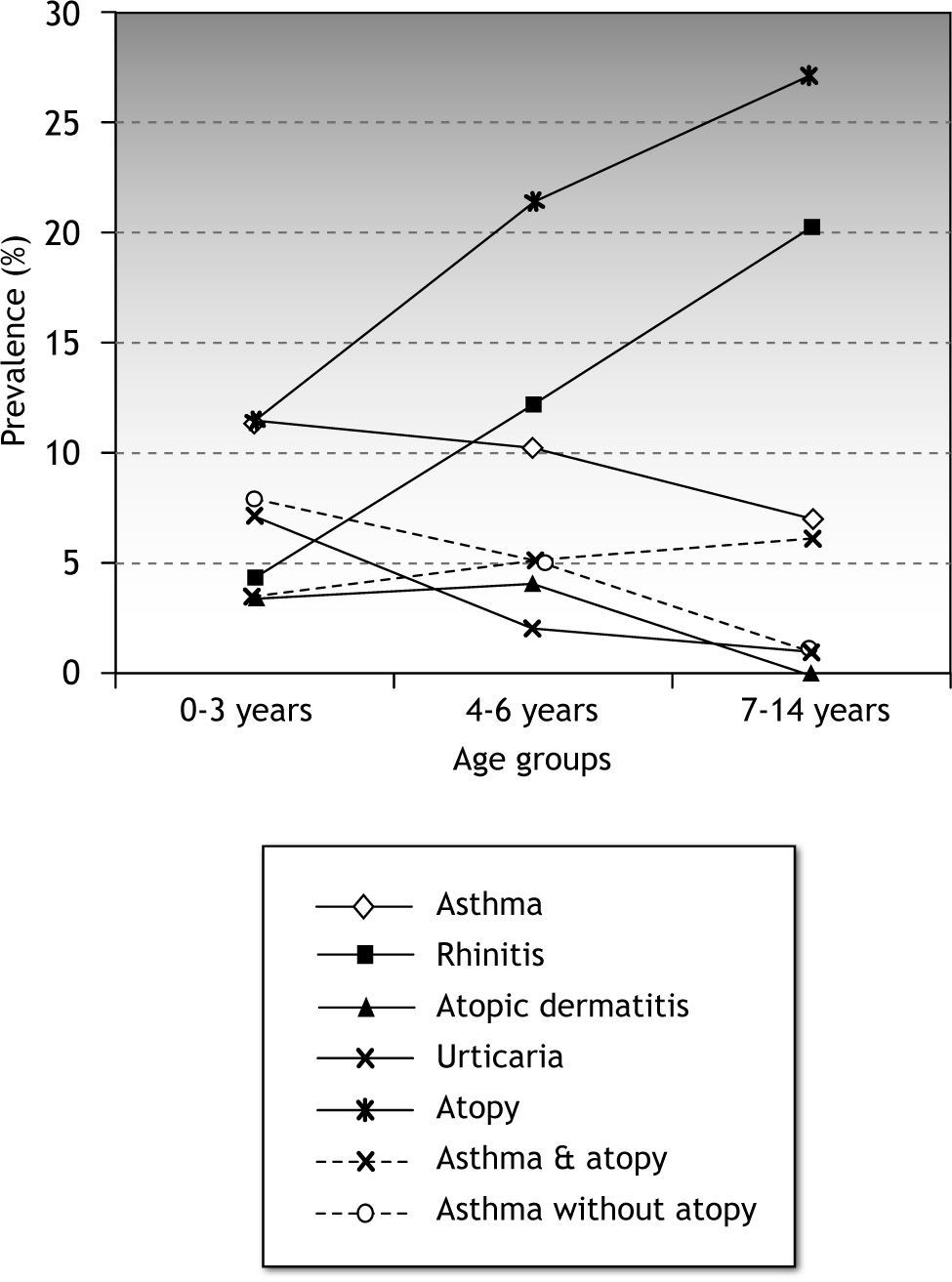
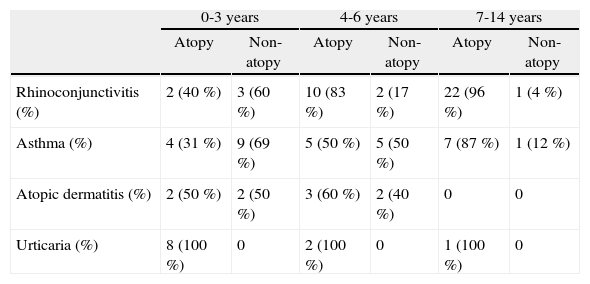
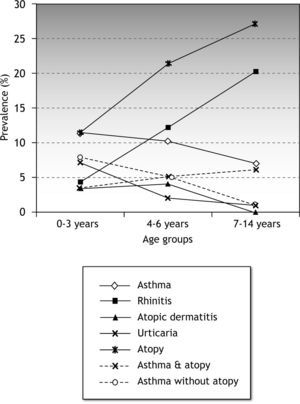
Any atopy-related disease was present in 83/325 (25.5 %; IC95 %: 20.6-30.4) children less than 15years. Prevalence of ARD increased with age (Fig. 1). This increase was associated with a sharp increase in allergen sensitisation among children with ARD. Allergen sensitisation in children with ARD changed from 42.3 % and 65.4 % in the 0–3 and 4–6 age groups, to 93.3 % in the 7–14 age group (p < 0.0001). Differences in the prevalence of atopy among children with atopy related diseases are shown in Table II.
Distribution of atopy related diseases by age groups and allergen sensitisation
| 0-3years | 4-6years | 7-14years | ||||
| Atopy | Non-atopy | Atopy | Non-atopy | Atopy | Non-atopy | |
| Rhinoconjunctivitis (%) | 2 (40 %) | 3 (60 %) | 10 (83 %) | 2 (17 %) | 22 (96 %) | 1 (4 %) |
| Asthma (%) | 4 (31 %) | 9 (69 %) | 5 (50 %) | 5 (50 %) | 7 (87 %) | 1 (12 %) |
| Atopic dermatitis (%) | 2 (50 %) | 2 (50 %) | 3 (60 %) | 2 (40 %) | 0 | 0 |
| Urticaria (%) | 8 (100 %) | 0 | 2 (100 %) | 0 | 1 (100 %) | 0 |
The mean prevalence of asthma was 9.5 % (IC95 %: 6.2-12.9). This changed through age groups, slowly decreasing from 11.5 % in the 0-3-year group to 10.2 % and 7 % in the 4–6 and 7-14-year age groups, respectively. The prevalence of atopy in the asthmatic children, 51.6 % (IC95 %: 32.4-70.8), increased through the age groups from 31 % in the 0–3 age group to 88 % in the 7–14 age group (Table II). Rhinoconjunctivitis was associated to asthma in 12.9 % (IC95 %:3.6-29.8) of the asthmatic children. By age groups, rhinoconjunctivitis was associated to asthma in 7.7 %, 10 % and 25 % in the 0-3-year, 4–6 and 7-14-year age groups, respectively.
RhinoconjunctivitisOverall, the prevalence of rhinoconjunctivitis was 12.3 %. A marked increase of rhinoconjunctivitis occurred through age groups, increasing from 4.4 % in the 0-3-year group to 20 % in the 7-14-year group (Table II). Prevalence of atopy among children with rhinoconjunctivitis also increased. Atopy rose from 40 % in the 0–3 age group to 96 % in the 7–14 age group.
Atopic dermatitis and urticariaThe prevalence of atopic dermatitis and urticaria decreased through the age groups as shown in Figure 1. All the children with urticaria had a positive skin prick test (Table I).
DiscussionPrevalence of atopyThere are few studies of prevalence of atopy in general paediatric populations. In this study, overall atopy prevalence was found in 20 % of children less than 15years of age. Similar reports have been observed in Germany (20.5 %)2 and Ecuador (18.2 %)11. However, our figures are in the low range of the rates so far reported4,6,12–14. Comparing results of atopy prevalence by age groups we observed that atopy prevalence (11.5 %) in the 0–3 age group was not distant from the 10.5 % observed in a study performed by Claver in the mid-eighties with the same group age and population15. This suggests that the prevalence of atopy has not changed in our population in the last three decades. In the same way, the prevalence of atopy in the 4–6 and 7–14 age groups are similar to others already published8,11,16.
In the last years, the studies performed to evaluate the prevalence of atopy and atopy-related diseases have been usually based on the ISAAC methodology5 in addition to SPTs2,8,12,13. However, the studies using the ISAAC methodology have reported very different atopy rates, ranging from 9 % in Poland3 to 57.7 % in Hong Kong13. Furthermore, during the last decades an increase in the prevalence of atopy has been reported6–8. Both changes and differences in atopy prevalence could be the result of the variations in the genetic constitution, but also to environmental factors. Nevertheless, environmental changes or shifts in the genetic fingerprints are very unlikely to explain any increase in prevalence during this time since they need to be fast enough to favour this increment. Migratory movements have also been suggested as responsible for this change but there are not enough data to support this hypothesis3.
Accuracy of the study methodologyThe high extent of discrepancy among the data so far reported encouraged us to carry out this study using a different methodology. The methodology used in most of the previous studies has great difficulty to achieve a high rate of enrolment and are also time consuming for many departments. Using patients referred to outpatient offices as a sample for atopy prevalence studies could avoid these problems if they account for the general population. Appointments for possible allergic reactions to drugs are very frequent in childhood. In most cases viral reactions are the cause of these exanthematic or urticariform reactions and diagnoses of drug allergy are not usually confirmed. As there is no evidence that suggests any relationship between this type of reaction and atopy or atopy-related diseases, this might be a suitable sample. In our hospital it accounts for 9 % of all the children referred to the allergy department, but only in 9 % of them the allergy suspicion was confirmed. The rest (91 %) are probably viral reactions without any IgE mediated mechanism. For this reason, this sample can be considered representative of the general children's population and is ideal to perform prevalence studies. On the other hand, a doctor's diagnosis of any atopy-related disease is more accurate than that obtained from questionnaires. It is also time saving for the physicians who directly evaluate the patients.
Asthma and atopy-related diseasesReported prevalence of atopy-related diseases in this study is in accordance with the literature. Prevalence of asthma reported in our study was in the low range of that reported in other studies in Spain17–19. Differences with some studies are probably due to the variability of data existing on this subject. In our study, the prevalence of atopy among asthmatic children increased through the age groups at the same time that asthma in non-atopic patients decreased. These observations are similar to the Tucson asthma cohort10. Most of the previous reports of asthma prevalence are based on questionnaires or/and exercise-induced bronchial responsiveness20–22. In our study, prevalence of asthma was based on doctor's diagnosis of asthma. Doctor's diagnosis of asthma seems to be more precise than a questionnaire or a questionnaire with exercise-induced responsiveness because it combines a physician's direct anamnesis with a group of diagnostic tools (pulmonary function, exercise-induced bronchial responsiveness, exhaled nitric oxide, etc). For this reason, the prevalence of asthma in our study should be as accurate, if not more so, than the previous reports.
Overall prevalence of rhinoconjunctivitis was 12.3 %. Prevalence per age group was similar to two recent reports from Spain18,23. The increase in the prevalence of rhinoconjunctivitis was parallel to the increase of atopy (Fig. 1) in the sample. In a similar way as occurs in asthma, the prevalence of atopy among patients with rhinoconjunctivitis also increased through age groups.
Differences with ISAAC methodologyAs previously discussed, previous reports studying prevalence of atopy were mainly based on ISAAC methodology and presented very different atopy rates. Interestingly, many of these studies have the same possible methodological shortcomings, i.e., they have a low rate of prick tests in the study samples if compared to the total number of patients invited to participate in the study4,12,14,24. It is possible that this methodological inaccuracy leads to a selection bias and explains the disparity observed in these reports of atopy prevalence. A selection bias could have been made because parents with children with atopy-related diseases would be more prone to having an SPT done on their children, increasing the number of individuals with atopy in their samples. Differences in the rates of prick test performed are very high among the studies3,4,11,24,25. They range from as high as 88 % in Ecuador,11 to the very low 34 % in Spain12. For this reason, studies with high rates of SPT, would usually result in a lower prevalence of atopy and vice versa3,4,11. In a similar way, some studies which indicate a rise in the prevalence of childhood atopy, could also have the same inaccuracy. For instance, a study carried out by Downs et al. in Australia6, reported an increase in atopy, but they also had a decrease in the response rate to questionnaires and SPT in the study period, with higher prevalence of atopy. However, in studies like the one performed by Grize et al26, where questionnaire response rates remained stable over the time, no trends towards an increase was observed.
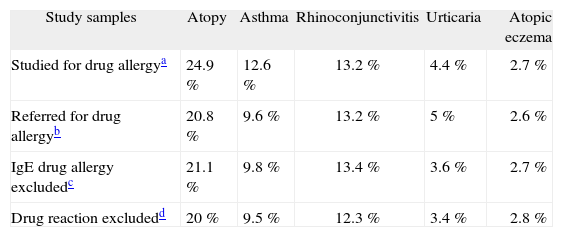
Possible biases and limitation in our study (internal validation)The main limitation of our study is that our design is based on data obtained retrospectively. Another limitation was our sample size. Although for general data the sample size was sufficient when divided in groups the precision dropped to 8.8 % in some cases. In this study we analysed some possible biases. First, we analysed if significant changes occurred when all children referred for drug allergy study were included, obtaining a negative result. Second, we analysed data excluding only children with a positive drug allergy but including those for a positive result to non-steroid anti-inflammatory drugs (NSAIDs). Intolerance to NSAIDs is considered to be produced by non IgE-mediated mechanisms. In our report we decided to exclude these patients because the prevalence of atopy among children with NSAIDs is not well established. However, when included in the analyses no differences were observed. Lastly, when analyses were made including all patients studied in the department for drug allergy, referred or not for this reason, as expected a higher prevalence of asthma and atopy was observed. We considered that including children studied for other reasons in our department would represent a bias because of the higher prevalence of atopy among children followed-up in our department. Differences observed are summarised in Table III.
Differences in prevalence of atopy related diseases with different study samples
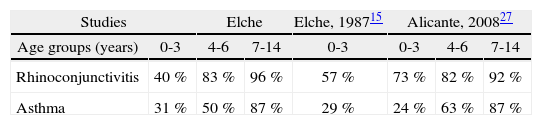
External validation was performed comparing differences with reports from the same region. In Table IV, differences in atopy prevalence among children with asthma or rhinoconjunctivitis was compared with studies performed in Elche15 and Alicante27. Only minimal differences were observed. Prevalence of atopy-related diseases in our study did not differ with previous studies in our region. No significant differences in prevalence were found with Claver's study15 (0 to 3-year old children) in asthma (11.5 % vs 10 %), rhinoconjunctivitis (4.4 % vs. 4.5 %), urticaria (7 % vs. 5.5 %), and atopic dermatitis (3.5 % vs. 3 %). In the same way, no important differences in asthma can be observed between our 7 to 14-year age group and the 6 to 7-year age group in a study performed in Valencia (7 % vs. 9 %)28. For this reason, we consider that external validation was achieved.
ConclusionWhen prevalence of atopy and atopy-related diseases are studied, reports are very different. This fact limits the possibility of extrapolating data to local areas and reinforces the need for more studies. Children remitted to an allergy office to discard an allergic drug reaction can be considered representative of the general population. Prevalence studies based on the analyses of these children can be a suitable sample.