The majority of allergic asthmatic patients are sensitised to several aeroallergens. Discrimination of the clinically relevant allergen is essential for the correct use of immunotherapy.
ObjectiveTo investigate nasal challenge and its role in screening clinically relevant allergens in asthmatic children.
MethodsAeroallergen nasal challenge was performed in five different groups of patients (asthma; asthma & rhinitis; rhinitis; atopic controls; and non-atopic controls). Differences between groups after challenge were evaluated by means of spirometry and acoustic rhinometry.
ResultsNasal challenge was performed in 125 patients, 25 per group. The positive nasal response of immediate type was recorded in 21 patients with asthma only (P<0.001), 18 with asthma and rhinitis (P<0.001), 19 with rhinitis (P<0.001), two atopic control patients and in no healthy control patients. However, no differences were observed between the asthma group and the groups with rhinitis symptoms. The risk of a positive challenge was much higher in the asthma without rhinitis group compared to atopic controls (OR 29.57; 95%CI: 5.47–159.97).
ConclusionAeroallergen nasal challenge is a safe technique in asthmatic children and could be useful in establishing the clinically relevant allergen even in the absence of rhinitis.
In 1998 the World Health Organization recommendation on the use of aeroallergen immunotherapy was that allergen immunotherapy is indicated for patients who have demonstrable evidence of specific IgE antibodies to clinically relevant allergens, and whose allergic symptoms warrant the time and risk of allergen immunotherapy.1 However, in common practice it is often difficult to determine the clinically relevant allergen in children sensitised to multiple aeroallergens. In a study performed in the United States, 80% of allergic asthmatic adults were sensitised to more than one aeroallergen.2 This high prevalence of multiple sensitisations in asthmatic patients suggests that the prediction of immunotherapy efficacy is even more complicated.
In the case of children with rhinitis, the use of nasal aeroallergen challenge can be an objective technique to determine the clinically relevant aeroallergen amongst the detected sensitisations.3 We carried out a prospective study in order to evaluate the effect of nasal challenge in asthmatic children without rhinitis. The main purpose of this study was to measure the changes in the nasal mucosa and pulmonary function after a nasal challenge with the suspected relevant allergen in this group of children.
MethodsA prospective study was conducted to evaluate the impact of nasal challenge with aeroallergens in asthmatic children at Elche General University Hospital (Alicante, Spain). Consecutive children who visited our outpatient clinic and met the inclusion criteria were invited to participate: to a total of 25 patients per group. Inclusion criteria were age (5–16 years of age) and presenting one of the following conditions: 1) healthy children without allergen sensitisation (control group); 2) healthy children with aeroallergen sensitisation (atopic control group); 3) patients with allergic rhinitis (rhinitis group); 4) patients with allergic asthma and rhinitis (asthma and rhinitis group); 5) patients with allergic asthma without rhinitis (asthma group).
Current asthma was defined as a department doctor’s diagnosis and included a documented history of episodes of wheezing and/or chest tightness, or a history of asthmatic symptoms with a positive response to a bronchodilator,4 within the last year. Rhinitis diagnosis required frequent or seasonal symptoms with two or more of the following: itchy nose, rhinorrhoea, sneezing, and nasal blockage. The diagnosis of rhinitis and asthma in the same patient required the fulfilment of the aforementioned criteria for asthma and rhinitis. The atopic controls were defined by the prick as subjects sensitised to ≥1 aeroallergen, without rhinitis or asthma. Healthy controls were defined as subjects without asthma, rhinitis, or any allergen sensitisation. These last groups of children had been studied in our clinic for other reasons, generally drug allergy, and after being studied, no allergic disease, asthma or rhinitis were diagnosed. All subjects had at least one screening with the prick test using the most common allergens in our area. Patients with asthma and/or rhinitis had a more extensive sensitisation study (using an extended panel of allergens by prick and/or RAST), as well as a calendar of symptoms when needed. Patients who did not meet the aforementioned conditions, were <5 or >16 years of age, or had other chronicle non-allergic diseases, were excluded from initial selection.
Skin prick test was performed with a panel of the most common aeroallergens (ALK-Abello, Spain): Dermatophagoides pteronyssinus, Dermatophagoides farinae, Blatella germanica, cat dander, dog dander, rabbit dander, horse dander, Alternaria, Aspergillus, Olea europaea, Artemisia, Parietaria judaica, mixed grasses (Dactylis, Lolium, Festuca, Poa, Phleum and Avena) and peach. Histamine (10mg/ml) and saline solution (0.9%) were used as positive and negative controls, respectively. When other allergen sensitisations were suspected they were added to the allergen panel. Skin prick tests were performed by an expert nurse with ALK-Abello (Madrid, Spain) lancets. A child was considered to be sensitised to a specific allergen if a wheal diameter measuring 3mm or more (after subtraction of the control value) was observed 15min after the prick.
Nasal challenge was performed with that aeroallergen suspected as being the most clinically relevant. Aeroallergen clinical relevance was based on clinical data (such as seasonality of the symptoms, a calendar of symptoms performed by the patient, regional aerobiology data or those suspected triggers), prick wheal diameter and/or allergen RAST levels. In the atopic control group nasal challenge was performed on one of the aeroallergens to which the patient was sensitised, alternatively selecting one of the three predominant aeroallergens in our region (Salsola kali, Alternaria alternata and Dermatophagoides pteronyssinus). In the non-atopic control group, Salsola kali, Alternaria alternata and Dermatophagoides pteronyssinus allergen solution were used alternately.
During the study any sprayed nasal corticosteroid and H1-receptor antagonists were withheld for seven days prior to the nasal challenge. On the day of the test a spirometry and an exhaled nitric oxide (FeNO) measurement was performed on all participants. Immediately afterwards, a nasal challenge with aeroallergens and nasal rhinometric measurements was performed. Spirometry was measured again 30min after the last challenge. Patients were requested to communicate to the departments’ staff if there was any exacerbation in asthma symptoms during the following 24 hours.
FeNO was measured online according to the recommendations of the ERS/ATS standard using a nitric oxide analyser (NIOX-MINO, Aerocrine, Sweden).5–8 Spirometry was performed with a conventional spirometer, Datospir 120 (Sibelmed, Spain), according to the ATS/ERS recommendations.9,10 A fall in FEV1 of 10% or more was considered abnormal.
The challenge was started by spraying the dilution media in both nostrils, followed by increasing the concentrations of the allergen extract (1:100, 1:10 and 1:1 lastly). Subjects were asked not to inhale with the nose during the allergen spraying. After 15min, the response was measured and the next allergen dilution was sprayed. Nasal response was measured by acoustic rhinometry using a SRE 2000 rhinometer (Interacoustics, UK). A positive nasal response was defined as a 30% fall in the cross-sectional volume in the second nasal notch (CSV2), with regard to the baseline obtained by spraying an allergen-free dilution11,12 Nasal challenge was performed with 10BU/ml concentration extracts from Alk-Abello for Dermatophagoides pteronyssinus, Olea europea, Salsola kali, cat dander, grass mixture and Alternaria alternata. Dilutions were performed with 0.5% phenol (ALK, Spain) to obtain 0.1, 1 and 10BU/ml concentrations of the different extracts.
Before starting, the study was approved by the department’s ethical committee. Informed consent was obtained from the rest of the participants. Data was processed using a Microsoft Office Excel database. Statistical analysis was performed using Biostat 5.1.3 package software (AnalystSoft). Data distribution is shown as mean±SD (or range), as indicated. FeNO values were log transformed for analysis and transformed back into ppb. Comparisons between groups of CSV2 differences were studied using Mann-Whitney U-test. Challenges between groups were analysed using the chi-square association. For intra-group analysis of spirometric values a Wilcoxon signed-rank test was used. A value of P<0.05 was considered to be statistically significant.
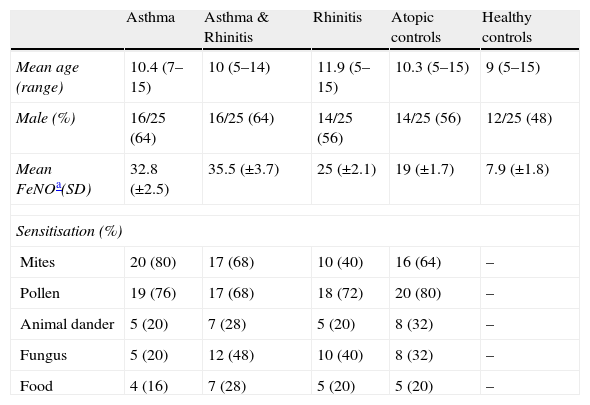
ResultsA total of 125 children, 25 per group, were enrolled in this study. Distribution among the study groups was homogeneous for age (mean 10.3 years, range 5–15) and sex (58% male). The most common sensitisations were to pollen (74/100) and mites (63/100). General group characteristics are summarised in Table 1.
Main characteristics of the sample groups
| Asthma | Asthma & Rhinitis | Rhinitis | Atopic controls | Healthy controls | |
| Mean age (range) | 10.4 (7–15) | 10 (5–14) | 11.9 (5–15) | 10.3 (5–15) | 9 (5–15) |
| Male (%) | 16/25 (64) | 16/25 (64) | 14/25 (56) | 14/25 (56) | 12/25 (48) |
| Mean FeNOa(SD) | 32.8 (±2.5) | 35.5 (±3.7) | 25 (±2.1) | 19 (±1.7) | 7.9 (±1.8) |
| Sensitisation (%) | |||||
| Mites | 20 (80) | 17 (68) | 10 (40) | 16 (64) | – |
| Pollen | 19 (76) | 17 (68) | 18 (72) | 20 (80) | – |
| Animal dander | 5 (20) | 7 (28) | 5 (20) | 8 (32) | – |
| Fungus | 5 (20) | 12 (48) | 10 (40) | 8 (32) | – |
| Food | 4 (16) | 7 (28) | 5 (20) | 5 (20) | – |
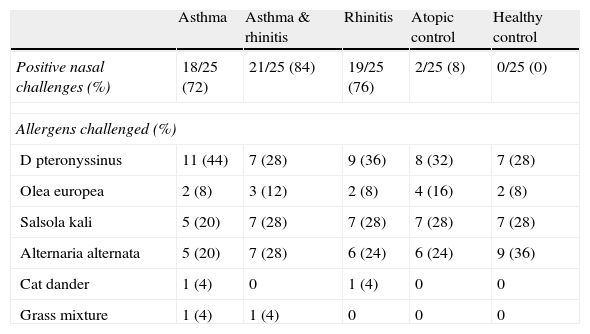
Overall, 80/125 (64%) had a positive nasal response to the aeroallergen challenge at 10BU/ml. The number of positive nasal challenges is analysed by groups in Table 2. Statistically significant differences were observed in the number of positive challenges at 10BU/ml between control groups and the rhinitis; rhinitis & asthma; and asthma groups (P<0.001). However, no statistically significant differences were found between these last three groups (rhinitis; rhinitis & asthma; and asthma). Even for the asthma group the risk of a positive challenge, compared to the atopic control group, was very high (OR 29.57; 95%CI: 5.47–159.97).
Number of positive nasal challenges and allergens employed
| Asthma | Asthma & rhinitis | Rhinitis | Atopic control | Healthy control | |
| Positive nasal challenges (%) | 18/25 (72) | 21/25 (84) | 19/25 (76) | 2/25 (8) | 0/25 (0) |
| Allergens challenged (%) | |||||
| D pteronyssinus | 11 (44) | 7 (28) | 9 (36) | 8 (32) | 7 (28) |
| Olea europea | 2 (8) | 3 (12) | 2 (8) | 4 (16) | 2 (8) |
| Salsola kali | 5 (20) | 7 (28) | 7 (28) | 7 (28) | 7 (28) |
| Alternaria alternata | 5 (20) | 7 (28) | 6 (24) | 6 (24) | 9 (36) |
| Cat dander | 1 (4) | 0 | 1 (4) | 0 | 0 |
| Grass mixture | 1 (4) | 1 (4) | 0 | 0 | 0 |
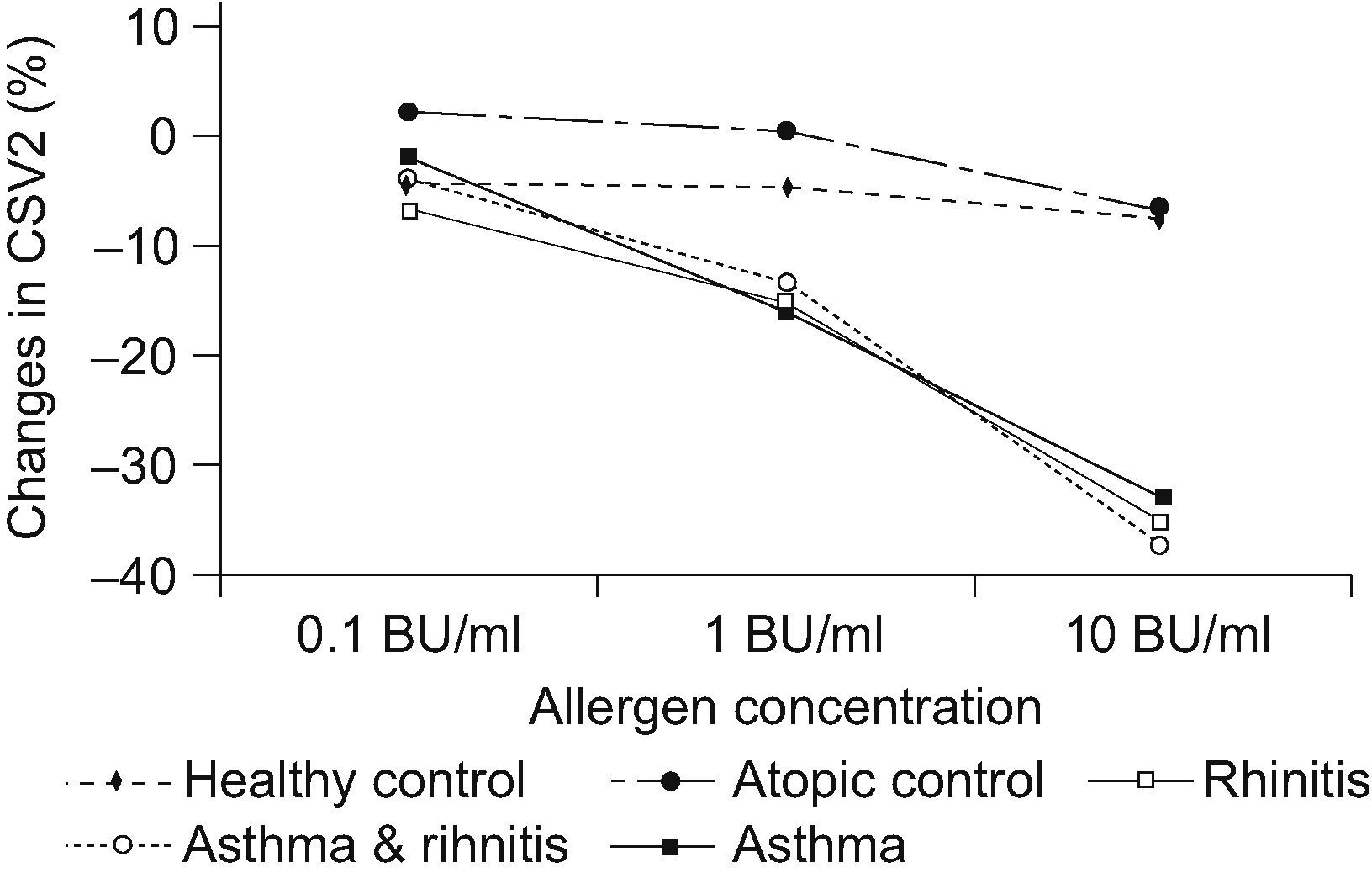
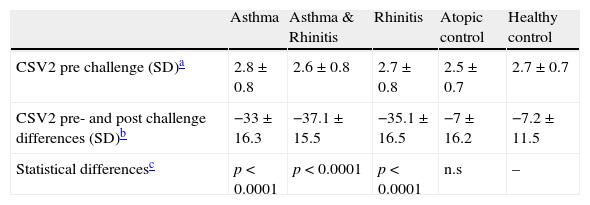
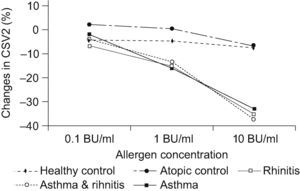
A statistically significant decrease (P<0.001) was found for the CSV2 at 10BU/ml allergen challenge in the rhinitis; rhinitis & asthma; and asthma groups compared to the control groups (See Table 3). Changes in CSV2 after nasal challenge were not statistically significantly different between rhinitis; rhinitis & asthma; and asthma groups. A significant decrease was also observed at 1BU/ml challenge in the same groups compared to controls but not at 0.1BU/ml allergen concentration (see Figure 1).
Differences in the mean volume of the cross-sectional area through the 2nd nasal notch (CSV2) after nasal challenge
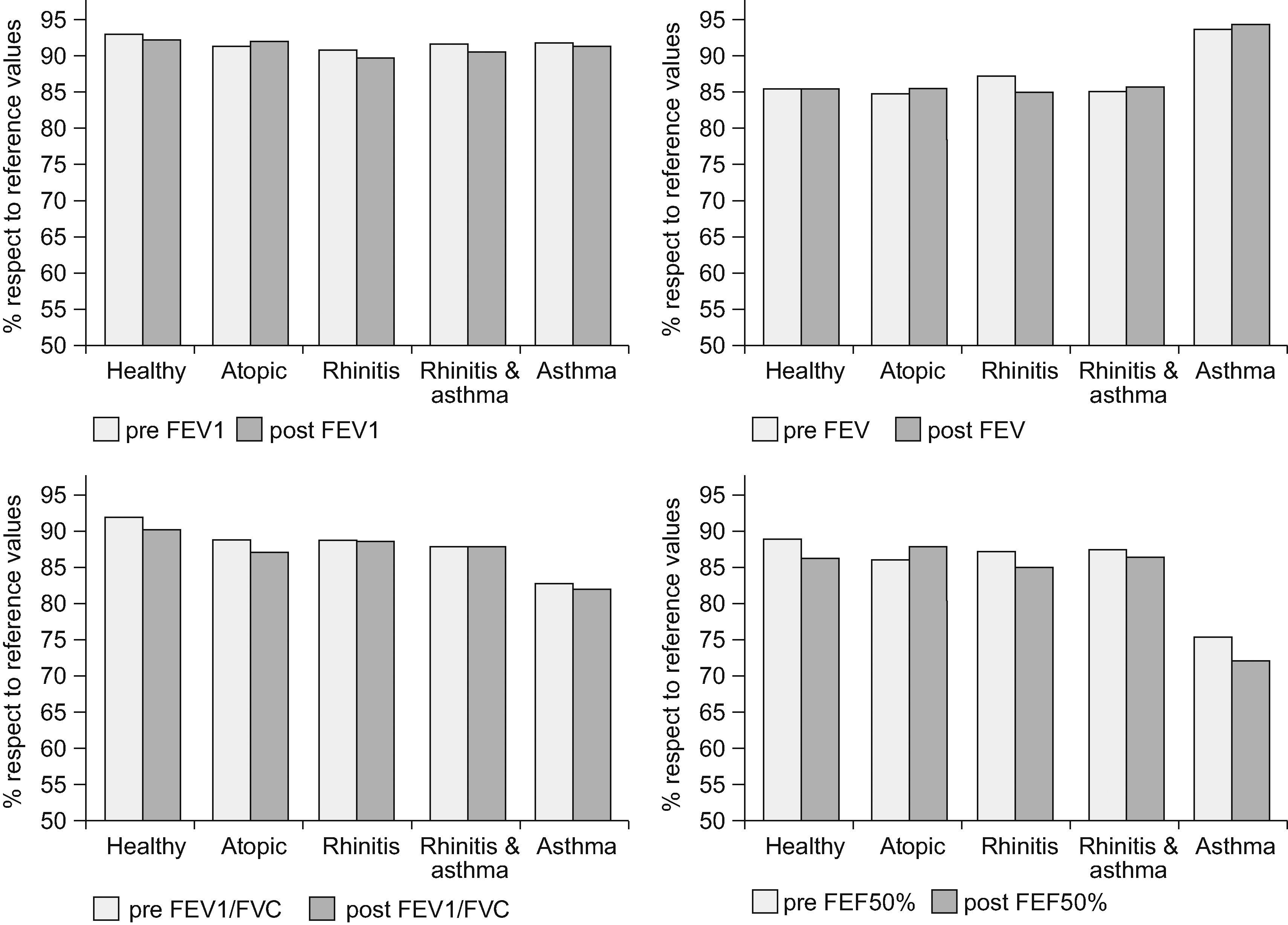
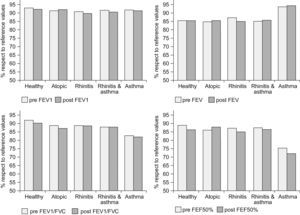
No significant differences were found in spirometric parameters after the nasal challenge in any of the study groups (see Figure 2). There were no significant differences after excluding negative nasal challenges either. Only one patient in the rhinitis & asthma group presented a 10% fall in FEV1 after the nasal challenge. Another child was attended 24 hours after the nasal challenge at the emergency department for an asthma exacerbation within a febrile viral infection. No other children reported asthma symptoms during the following 24 hours after the nasal challenge.
DiscussionDetermination of the role of aeroallergens in asthma is generally based on clinical history, aeroallergen sensitisation (measured by prick test or specific IgE) and allergen exposure.13 Occasionally bronchial challenges are performed to prove the suspected clinical role of the aeroallergen. Unfortunately, although bronchial challenge with the clinically relevant allergen is probably the gold standard to assess the real relevance of an allergen, this technique is very uncomfortable for patients, needs a direct control by the physician, and is not risk free for both immediate and long-term consequences.14,15 For these reasons, aeroallergen bronchial challenge is not frequently used in clinical practice and is usually employed for research purposes. On the other hand, there are increasing data which supports the unique airway theory that rhinitis and asthma are the same disease observed at different points of the air tree, sharing common aetiopathological mechanisms.16–18 That is the background by which we tested the hypothesis that a nasal challenge could produce a similar nasal response and/or spirometric changes in children with asthma compared to those with rhinitis.
In the present study, we have observed that children with asthma without rhinitis had a similar nasal response to those with allergic rhinitis. This similar response to the nasal challenge did not only depend on the atopic condition, as proved by the large differences in the response rate between the asthma group and the atopic control group (72% vs. 8%). The grade of response in the asthma group was similar to the other two groups with rhinitis. These findings suggest that patients with asthma without rhinitis can develop symptoms of rhinitis when a sufficient concentration of the allergen is reached. Probably the children with asthma who had a positive nasal challenge need a higher concentration of the allergen to develop nasal symptoms compared to the children with rhinitis. Concentrations used in an allergy laboratory are usually higher than those reached in the environment. The differences between the allergen concentrations used in the laboratory challenges and the environmental exposure could explain why these children do not usually show nasal symptoms.
Few studies have assessed the impact of nasal allergen challenge in asthmatic adults and we are not aware of any reports in children.19–22 The few reports on this topic have studied the spirometric and inflammatory changes after nasal challenge in asthmatic patients with symptoms of rhinitis but none have previously reported the nasal challenge response in asthmatics without rhinitis. Studies performed by Inal and Marcucci did not observe changes in the mean FEV1.18,19 Nonetheless, Marcucci reported that 3/15 patients had a 10% fall in FEV1. In our study only one out of 50 asthmatic patients presented a 10% fall in FEV1. This difference with Marcucci’s study might be due to our concern that the child did not inhale the allergen solution. Contrary to this study and ours, in which no significant changes were observed in pulmonary function, Pelikan observed a FEV1 decrease in 59/68 patients after nasal challenge. However, in that study nasal challenges were performed in patients with asthma and rhinitis who had previously had a negative response to the same allergen in a bronchial challenge. Pelikan suggests that these differences are due to the existence of a group of asthmatic patients whose asthmatic response is not secondary to the allergen direct bronchial triggering of the inflammatory cascade but to a nasal trigger.23 This could be in accordance to Marcucci´s and our own findings; mean pulmonary function would generally not be affected in an unselected asthmatic cohort except for the few cases where FEV1 decreased as triggered by the nasal mucosa.
Interestingly, we found that we erroneously identified the clinically relevant aeroallergen in 10/50 (20%) of the children with rhinitis. If the immunotherapy schedule had been prescribed, an absence of any beneficial effect would have been expected in this 20%. This reflects the difficulty in identifying the relevance of aeroallergens in asthma or rhinitis by anamnesis. The use of objective measurements to obtain aeroallergen relevance should be a more extended practice. In the case of rhinitis, nasal challenge with aeroallergens measured by acoustic rhinometry seems an optimal technique.24 On the contrary, the systematic use of aeroallergen bronchial challenge in clinical practice is controversial. For this reason our findings could be clinically relevant. The nasal response in asthmatic children without rhinitis observed in our study suggests that there could be a correlation between nasal and bronchial challenge. Supporting this hypothesis, Lopuhaä et al. reported in mite sensitised a similar bronchoconstriction after bronchial challenge with house dust mite extracts in both asthmatic and in rhinitic without asthma patients.25 The use of nasal challenge instead of bronchial challenge could be useful in certain asthmatic patients. Still, further studies to confirm or discard the correlation of nasal and bronchial challenge and its clinical relevance in asthmatic patients should be conducted.
Acoustic rhinometry is an objective technique to measure nasal response after aeroallergen challenge. However, it has still to be well standardised and the most useful parameters are not well established. In two previous studies we observed that the most accurate measurement in nasal challenge was obtained by measuring nasal volume through the second nasal notch. In those studies, measurement of the nasal volume through the second nasal notch was the best correlated to the symptoms after aeroallergen challenge. The best cutpoint was a 28% volume fall respect to basal.11,12 In order to simplify we decided to establish a 30% fall in nasal volume as positive. This practically corresponded to two standard deviations (29.6%) of the nasal volume fall measured through the second notch at 10BU/ml allergen challenge in the non-atopic control group (data not shown). In our experience, the maximum decrease in CSV2 is observed 15min after nasal challenge and for this reason this point was introduced in the study protocol. Interestingly, this corresponds to the maximum diameter of prick test wheals.
Our study has several limitations. First of all, the selected cohort comprised mainly polisensitised patients. This fact was probably responsible for the failure to identify the clinically relevant aeroallergen and the subsequent negative response to the nasal challenge in 20% of the patients with rhinitis. Secondly, pulmonary function was not measured in the days following the challenge to discard a late phase response. Nevertheless, the fact that there were no direct symptoms after 24 hours of the challenge suggests that aeroallergen nasal challenge is a reasonably safe and well-tolerated technique in asthmatic patients.