Allergen-specific immunotherapy is a proven, highly effective treatment for IgE–mediated diseases. However, ultra-rush immunotherapy is prescribed infrequently because of the perception that accelerated immunotherapy buildup leads to a higher rate of systemic reactions.
ObjectiveTo evaluate the frequency of adverse reactions in patients with IgE–mediated diseases receiving house dust mite (HDM) ultra-rush immunotherapy.
MethodsA retrospective, observational study was conducted for patients with IgE–mediated diseases receiving allergen-specific immunotherapy. Subcutaneous immunotherapy with depigmented polymerized mites extract was administered in two refracted doses of 0.2 and 0.3ml at first injection, and in single 0.5ml doses in subsequent monthly injections. A 30min observation time was required after each injection. Systemic reactions were graded using the World Allergy Organisation grading system.
Results575 patients were included. The age range was 1–83 years. Most patients had respiratory diseases (544) and 101 patients had atopic dermatitis. A total of 27 patients (4.6%) experienced 139 reactions (reactions/injections: 1.9%); 22 patients (3.8%) experienced 134 local reactions (local reactions/injections: 1.8%). Eight patients (1.3%) experienced eight systemic reactions (systemic reactions/injections: 0.1%). Five systemic reactions were grade 2 and three grade 1. Two systemic reactions were reported during buildup. There were no fatalities.
ConclusionTaking into account the possible bias for the retrospective design of this study we observed that immunotherapy for patients with IgE–mediated diseases using a depigmented polymerized mites extract, with an ultra-rush buildup, has similar frequency of systemic reactions than that seen in slower buildup immunotherapy in other studies. Accelerated buildup could improve patients’ adherence and reduce dropout rates.
One of the principal factors cited against the widespread adoption of subcutaneous immunotherapy (SCIT) for asthma and other allergic diseases is the risk of serious adverse reactions.1 In the 1980s a review study reported incidence of systemic reactions in patients receiving SIT for asthma over 30%2 but in the last 20 years the prevalence of systemic reactions has been reported from 0.25% to 4%.3–6 When differential risks exist between therapies, the more risky therapy can only be justified if that therapy offers substantial additional benefit over the safer therapy. Allergen immunotherapy is the only treatment that controls clinical symptoms and simultaneously modified the course of allergic diseases like asthma, rhinitis, conjunctivitis and atopic dermatitis.
The Word Allergy Organisation (WAO) has been making an effort to unify the definition and classification of systemic reactions using five steps according to the system affected and the severity of the reaction;3 this could be useful for a homogeneous classification between studies and to evaluate possible risk factors such as the type of extract,7–9 immunotherapy schedule,10 and atopic disease treated.11,12
Slow buildups with several injections per week for two or three months are frequently used to avoid systemic reactions and some articles support a reduction of incidence with slow buildups compared with accelerated buildups when aqueous extracts are used.13,14 However, slow buildups have a higher drop-out rate and there are no studies evaluating if slow buildups are better than accelerated buildups when depigmented and polymerized extract are used. Here we present the results of a retrospective study with 575 patients evaluating the safety of IT with a depigmented and polymerized mites extract with a buildup phase of two injections in one day.
MethodologyThis retrospective study was designed to evaluate local and systemic reactions after immunotherapy with house dust mites (HDM) during the buildup and/or maintenance dosing. The study was conducted in a single allergy centre with six allergists associated at the University of Antioquia and was approved by the University Institutional Review Board.
Patients receiving SCIT for the period of May 2007–September 2011, were included. Subcutaneous Immunotherapy with depigmented polymerized mites extract (Leti, Madrid Spain) was administered monthly. Mite allergen extracts were administered in two refracted doses of 0.2 and 0.3ml during buildup, and in single 0.5ml doses (50 DPP) in subsequent monthly injections (Table 1). A 30min observation time was required after each injection, for observing and counteracting possible side effects. Patients or patients’ parents were instructed to identify and report any delayed reaction.
In our population we usually do immunotherapy against a single source of allergens, principally dust mite. In polysensitized patients with two or more sources that prove to be clinically relevant, we vaccinated with those extracts separately, however this is very infrequently and only nine patients of this group needed it.
To classify systemic reactions, the World Allergy Organisation subcutaneous immunotherapy grading system was used. The reactions of patients and the treatment provided were recorded at the time of the reaction taking into account the type of reaction (local, systemic), symptoms, time, organ systems affected.15
The clinical history of patients was reviewed for pertinent historical information. Particular attention was focused on sensitisation pattern (monosensitized, polysensitized) and allergic diseases.
ResultsPatient characteristicsFive hundred and seventy-five patients received ultra-rush mite immunotherapy. Patients with HDM immunotherapy had a mean age of 15 years with a mode of 10 and ranged from 1 to 83 years of age. Two hundred and ninety-four (51%) patients were female; all patients had an IgE-mediated disease diagnostic by an allergist (Table 2). Five hundred and forty-four (94.6%) patients had a respiratory disease; allergic asthma (313=54.4%) or rhinitis (505=87.8%). Two hundred and fifty-one (43.6%) had allergic conjunctivitis and 101 (17.5%) atopic dermatitis.
Three patients with HDM immunotherapy received dog dander immunotherapy too. Among the patients receiving mites, 541 were vaccinated with a combination of Der f/Der p; 13 with Blo t/Der f/Der p; 4 with only Der f; 10 with only Der p, and 7 with only Blo t.
ReactionsSeven thousand two hundred and fifty-six injections with HDM extract were registered; 725 buildup and 6533 maintenance injections. One hundred and twenty-three patients had two or more buildup because immunotherapy was suspended for more than three months. Twenty-seven (4.6%) of 575 patients who received HDM ultra rush immunotherapy experienced a local or systemic reaction, with a total of 139 reactions (reactions/injections: 1.9%).
Local reactions (LR)Twenty-two patients (3.8%) experienced 134 local reactions (local reactions/injections: 1.8%); 133 were hives and/or erythema and one had pain for three days. Most local reactions were during the first eight months of immunotherapy and in most patients these symptoms did not appear again after the eighth dose. However six patients presented local hives even after 50 injections.
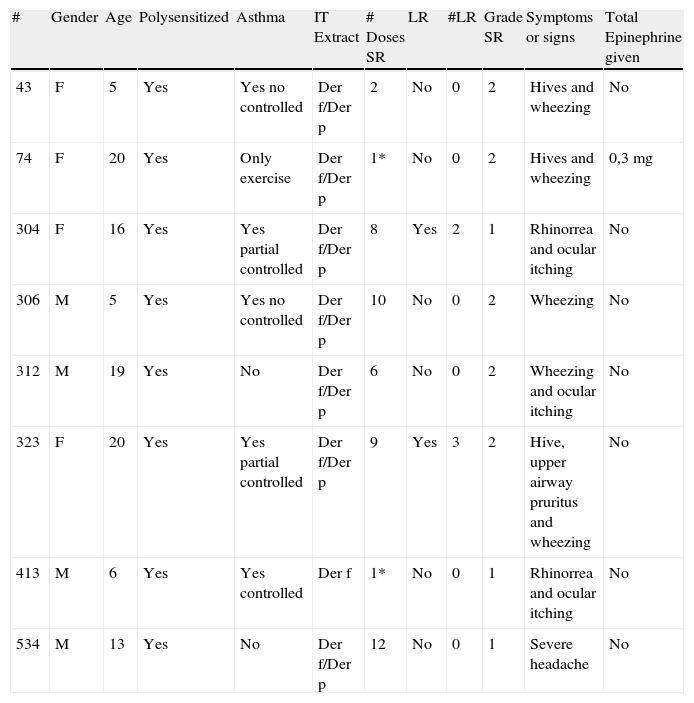
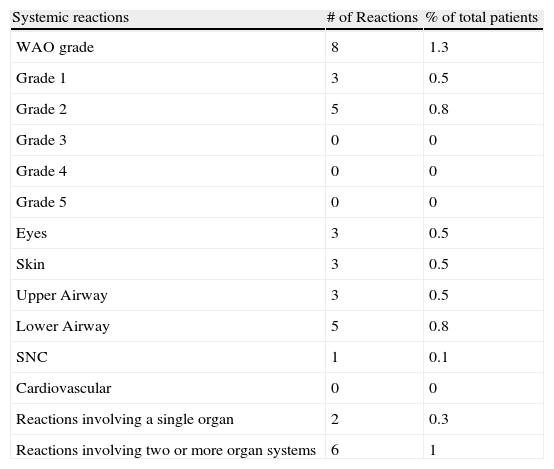
Systemic reactions (SR)Based on the WAO subcutaneous immunotherapy systemic reaction grading system, eight patients (1.3%) with HDM ultra rush immunotherapy had a total of eight systemic reactions (SR/injections: 0.11%) (Table 3); five grade 2 and three grade 1. Two reactions were during buildup (SR/buildup injections: 0.27%).
Systemic reactions.
| # | Gender | Age | Polysensitized | Asthma | IT Extract | # Doses SR | LR | #LR | Grade SR | Symptoms or signs | Total Epinephrine given |
| 43 | F | 5 | Yes | Yes no controlled | Der f/Der p | 2 | No | 0 | 2 | Hives and wheezing | No |
| 74 | F | 20 | Yes | Only exercise | Der f/Der p | 1* | No | 0 | 2 | Hives and wheezing | 0,3mg |
| 304 | F | 16 | Yes | Yes partial controlled | Der f/Der p | 8 | Yes | 2 | 1 | Rhinorrea and ocular itching | No |
| 306 | M | 5 | Yes | Yes no controlled | Der f/Der p | 10 | No | 0 | 2 | Wheezing | No |
| 312 | M | 19 | Yes | No | Der f/Der p | 6 | No | 0 | 2 | Wheezing and ocular itching | No |
| 323 | F | 20 | Yes | Yes partial controlled | Der f/Der p | 9 | Yes | 3 | 2 | Hive, upper airway pruritus and wheezing | No |
| 413 | M | 6 | Yes | Yes controlled | Der f | 1* | No | 0 | 1 | Rhinorrea and ocular itching | No |
| 534 | M | 13 | Yes | No | Der f/Der p | 12 | No | 0 | 1 | Severe headache | No |
Systemic reactions were principally airway symptoms (7/8) and cutaneous symptoms (3/8) (Table 4). All reactions were during the first 30min after the administration of immunotherapy. There were no delayed reactions. All patients with systemic reactions had rhinitis, and six had asthma. None had atopic dermatitis. There were no fatalities.
Reported symptoms during systemic reactions.
| Systemic reactions | # of Reactions | % of total patients |
| WAO grade | 8 | 1.3 |
| Grade 1 | 3 | 0.5 |
| Grade 2 | 5 | 0.8 |
| Grade 3 | 0 | 0 |
| Grade 4 | 0 | 0 |
| Grade 5 | 0 | 0 |
| Eyes | 3 | 0.5 |
| Skin | 3 | 0.5 |
| Upper Airway | 3 | 0.5 |
| Lower Airway | 5 | 0.8 |
| SNC | 1 | 0.1 |
| Cardiovascular | 0 | 0 |
| Reactions involving a single organ | 2 | 0.3 |
| Reactions involving two or more organ systems | 6 | 1 |
We did not pretreat with steroids or antihistamines, but 478 (83.1%) were receiving antihistamine daily as part of their pharmacology treatment for IgE-mediated diseases.
Risk factors for systemic reactionsWe observed some variables to identify possible risk factors of SR; the age of patients with SR was under 20 years (5–20). Six patients had asthma; three without control, two partially controlled, and one controlled. All patients with SR were polysensitized and were receiving antihistamines as part of the pharmacology treatment. Three patients with SR had local reactions previously.
SR treatmentOnly one patient with grade 2 SR received adrenaline. Oral antihistamines were given in each patient with SR. Two patients received intramuscular antihistamines. Two patients received intramuscular glucocorticoids and in three inhaled beta agonist was used.
Immunotherapy with other extractsDuring the study period only nine patients required immunotherapy with extracts different than HDM. Five patients received dog dander immunotherapy, one with cat at the same time. One patient received ants extract, two hymenopterans and one mosquito.
DiscussionControlled studies of immunotherapy usually conducted with single allergens, have demonstrated a reduction in respiratory symptoms caused by exposure to grass, cat, house-dust mite, ragweed, Cladosporium, and Alternaria.16–18 A meta-analysis of 88 randomised, placebo-controlled studies has confirmed the effectiveness of immunotherapy in asthma and rhinitis, with a significant reduction in asthma symptoms and medication and with improvement in bronchial hyper-reactivity.19 This meta-analysis included 42 trials for allergy to HDM, 27 for pollen allergy, and 10 for animal dander. On the other hand, only six trials with multiple allergen therapy which is commonly used in United States were included. Most of our patients received the mixed Der f and Der p for immunotherapy; both are mites from the same genus and have high cross-reactivity, however, each one has particular allergens that are not shared between species.Despite its proven benefits, only a small percentage of patients with allergic disease use immunotherapy, in part because of the inconveniences associated with treatment like risk of adverse reactions and because conventional buildup involves once or twice-weekly injections over the course of several months to reach the maintenance dose, requiring frequent visits to a physician's office and possibly time missed from school or work, which is the principal reason why many patients discontinue treatment before completing the recommended protocol.20–22Accelerated protocols of immunotherapy typically involve a faster buildup, reaching the maintenance dose in a few days or within a month, however these are prescribed infrequently because some studies have reported a higher incidence of reactions, nevertheless others have found no significant difference.14,23–27
Recently Christopher et al., in a multicentre study with 441 patients, reported that 10.9% of patients receiving cluster immunotherapy experienced a systemic reaction during buildup.28 As an explanation, they propose that the higher incidence of SR in the study is because it includes only patients with an aggressive buildup protocol consisting of eight visits (two per week) with increasing concentration of mites immunotherapy extract. In our study we did 725 ultra-rush mite buildup in 575 patients and only two presented a systemic reaction during this phase and six present SR during the maintenance phase; a very low frequency in comparison with other studies with accelerated protocols. In our protocol during the buildup we applied the maintenance concentration divided into two injections and patients had to wait for one hour. In the maintenance phase patients only have to wait only for 30min monthly. This protocol is more comfortable for patients and reduces the frequency of drop-out of immunotherapy or irregular assistance. Different factors could explain the low frequency of adverse reactions in our study compared with the report of Christopher and other articles: We used a depigoid hypoallergenic extract and we generally apply no more than one source of allergens, however it is necessary to be careful went comparing retrospective studies for possible bias. In articles with a retrospective design, some reactions could not be reported under the new classification of the WAO and it is difficult to compare different schedules. However, in our study all patients were clinically evaluated during immunotherapy and any changes in the patient were recorded so it is unlikely to have missed any reaction.
Several previous studies have been published demonstrating the safety of immunotherapy with modified extracts.29,30 Casanova et al. included in a prospective observational study 1068 patients with rhinoconjunctivitis and/or asthma sensitized to mites and/or pollen using the same schedule as us and they found five immediate systemic reactions and three delayed grade 2 reactions after 2136 injections (0.66% per patient and 0.4% per injection).31 The frequency of reactions per injections was slightly higher than found by us, however only one of the eight reactions was with mites; the other seven were with pollen extracts. Hernandez et al., evaluated the safety of immunotherapy after three years of therapy (1837 doses) in a group of 77 patients between two and five years and found only one systemic reaction (0.1%) using a cluster schedule (three days).32 Similar to the findings of Hernandez et al., in our group of children under five years (74 children) only two had a systemic reaction after doses of 859 (0.2%).
We explored potential risk or protective factors for SR described in other articles, which include gender, age, polysensitization, local reactions and premedication; these factors do not appear to be predictive of subsequent systemic reactions. However, the low incidence of SR in our study makes it difficult to determine a significant difference. We observed that all patients with SR were polysensitized and five had no control or partial control of asthma. So the main reason for SR in our study could be medical mistake.
Some articles report that 20%–30% of systemic reactions occurred after one hour of injection,33–35 however in our patients all adverse reactions occurred during the first 30min and we had no delayed reactions. Based on this finding, we considered that the observation period for 30–60min, as recommended by the American and European consensus of immunotherapy is as good for conventional buildup as for ultra-rush immunotherapy. It has been described that the principal reason for discontinued immunotherapy is large local reactions (18%), schedule conflicts (17%) or systemic reactions (24%).28 In our population none suspend immunotherapy for local or systemic reactions; the principal reason for stopping immunotherapy was problems with the social security system.
We have preliminary results that show a good clinical response with this ultra-rush immunotherapy schedule in patients with respiratory allergic diseases36 and even in patients with atopic dermatitis.37 Until recently, it was generally agreed that immunotherapy should not be used in patients with atopic dermatitis unless they have another respiratory atopic disease, but the third task force of the AAAAI consensus suggest the use of immunotherapy in patients with atopic dermatitis based on some studies of efficacy.3 However, the safety of immunotherapy in patients with atopic dermatitis has been little studied and some authors claim for precaution especially in patients with atopic dermatitis polysensitized with respiratory compromised. We evaluated HDM ultra rush immunotherapy in 101 patients with atopic dermatitis all of them polysensitized and 55 with asthma for a mean time of nine months per patient (one buildup nine maintenance per patient) and none presented systemic reactions. These results support the safety of immunotherapy in those patients with atopic dermatitis with or without asthma or polysensitisation.
In conclusion, mite immunotherapy for patients with IgE–mediated diseases using a depigmented polymerized mites extract with an ultra-rush buildup, has similar frequency of systemic reactions than that reported in slow buildup immunotherapy. On the other hand, ultra rush buildup could reduce the number of injections and thus improve patients’ adherence to treatment and reduce the drop-out rate.
Ethical disclosuresThis study was approved by the University of Antioquia (Medellin Colombia) Institutional Review Board.
Protection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all patients included in the study have received sufficient information and have given their informed consent in writing to participate in this study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestAuthors have no conflict of interest to declare.