Conventional immunotherapy for allergy with 3–5 years of treatment period has poor compliance. Ultra-rush sublingual immunotherapy with a shorter period of treatment can have better compliance. There are very few studies on ultra-rush sublingual immunotherapy all over the world.
Objectives(1) To determine allergen sensitivity among allergic rhinitis patients. (2) To assess safety, tolerability and clinical efficacy of ultra-rush sublingual immunotherapy.
MethodsThe present study was conducted in Allergy clinic, KIMS Hospital & Research Centre, Bangalore, India from January 2010 to June 2011. After obtaining Institutional Ethics Committee approval, 40 allergic rhinitis patients (according to ARIA guidelines) in the 18–60 years age group who were positive for aeroallergens in skin prick test were recruited for ultra-rush sublingual immunotherapy (20min initial phase and 4-month maintenance phase) and followed for 8 months with symptom and treatment diary.
ResultsOut of 40 patients, the majority, 36 (90.00%) patients were sensitive to house dust mites. Six patients had seven immediate adverse reactions and seven patients had eight delayed adverse reactions. All subsided without medication or with symptomatic oral medications. All patients tolerated ultra-rush SLIT and there was significant decrease in both symptom-score and treatment received in these patients.
ConclusionUltra-rush SLIT regimen has excellent safety, tolerability and clinical efficacy among allergic rhinitis patients.
Allergen-specific immunotherapy is the only treatment modality available for allergic diseases with proven long-term benefits. The traditional subcutaneous route is burdened with the risk of severe adverse events. Sublingual immunotherapy is a novel method, patient friendly, easy to administer, has fewer adverse reactions and is of equal efficacy compared to subcutaneous route.1 Sublingual immunotherapy (SLIT) regimens have traditionally an induction phase of updosing lasting approximately 2–3 weeks. Shorter regimens could simplify the administration and could be better accepted by patients, favouring their adherence to therapy.2 A metaanalysis in 2005 showed that frequency of adverse effects associated with SLIT was not dose-dependent.3 Local side effects are seen more frequently in the low dose groups, but no difference was seen in occurrence of systemic reactions.4 Ultra-rush sublingual immunotherapy with a shorter period of treatment can have better compliance. Recently ultra-rush regimens with induction phases lasting less than 2h have been tried.5–9 These studies have demonstrated the safety and tolerability of ultra-rush regimens in a few randomised placebo-controlled trials performed with cypress pollen5 and grass pollen6,7 and also in some observational studies.8,9 There are very few studies regarding the safety, tolerability and efficacy of ultra-rush sublingual immunotherapy all over the world, particularly in India. Nevertheless, additional and larger studies with other types of allergens are needed to further confirm the safety and efficacy of such regimens. Hence the present clinical trial was undertaken to study the safety, tolerability and clinical efficacy of ultra-rush sublingual immunotherapy among patients suffering from allergic rhinitis.
Objectives- 1.
To describe the socio-demographic profile of allergic rhinitis patients.
- 2.
To determine the allergen sensitivity among patients suffering from allergic rhinitis.
- 3.
To assess the safety and tolerability of ultra-rush sublingual immunotherapy based on adverse reactions.
- 4.
To assess the clinical efficacy of ultra-rush sublingual immunotherapy using symptom diary and treatment diary.
The study was conducted in Allergy Clinic, Preventive Medicine Unit, Kempegowda Institute of Medical Sciences Hospital and Research Centre (KIMSH&RC), Bangalore from January 2010 to June 2011 for a period of 18 months. This is a non-randomised clinical trial carried out on 40 allergic rhinitis patients sensitive to aero allergens with sensitivity levels of grade 2 and above.
Calculation of sample size:
| n=4pq/d2 |
| p–Prevalence of allergic rhinitis10=11% |
| q=1−p |
| With precision of 10% |
| n=4×0.11×0.89/(0.10)2=39.16 |
| Approximately 40 patients |
After obtaining the Institutional Ethical Clearance for the present study, individuals with clinical signs and symptoms of mild and moderate to severe persistent allergic rhinitis (as per ARIA guidelines11–13) were subjected to routine investigations such as Hb%, TC, DC, ESR and special investigations such as Modified Allergy Skin testing (Skin prick test), Absolute Eosinophil count, and Nasal smear for cytology.
Skin prick tests14–16 were performed with 123 allergen extracts. The extracts included 19 pollens, 5 dusts, 2 dust mites, 10 fungi, 10 insects, 3 epithelia and 74 food allergens. Allergen extracts for skin prick tests were obtained from Creative Drug Industries, Navi Mumbai. Pollen antigens were selected based on the pollen calendar.
Individuals who were sensitive for aero allergens with sensitivity levels of grade 2 and above17 in skin prick tests were included in the study and recruited for ultra-rush sublingual immunotherapy. Patients with autoimmune diseases, serious chronic inflammatory diseases, malignant disease, severe asthma, emphysema, bronchiectasis, pregnancy, ischaemic heart disease, high blood pressure, receiving immunosuppressive medications and beta-blockers, and suspicion of alcohol abuse were excluded.
Informed consent was obtained from each patient after informing about the nature and objectives of the study. The ultra-rush sublingual immunotherapy was given with an initial induction phase for 20min followed by a maintenance phase for 4 months where the patient had to take the maximum dose of 2ml (or tolerable dose) daily.
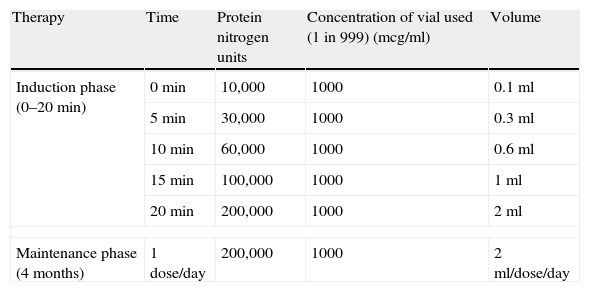
Customised vaccine kits (standardised allergen extract solutions) for ultra-rush SLIT were procured from Creative Drug Industries, Navi Mumbai based on the allergen sensitivity of the patients. The 20min ultra-rush sublingual immunotherapy protocol for induction phase was followed as per Gammeri et al.9 by converting the Allergoid Units standardised to w/v ratio/PNU estimation/biological activity test (according to the methodology of Allergo Pharma, West Germany), as shown in Table 1.
Ultra-rush sublingual immunotherapy protocol.
| Therapy | Time | Protein nitrogen units | Concentration of vial used (1 in 999) (mcg/ml) | Volume |
| Induction phase (0–20min) | 0min | 10,000 | 1000 | 0.1ml |
| 5min | 30,000 | 1000 | 0.3ml | |
| 10min | 60,000 | 1000 | 0.6ml | |
| 15min | 100,000 | 1000 | 1ml | |
| 20min | 200,000 | 1000 | 2ml | |
| Maintenance phase (4 months) | 1 dose/day | 200,000 | 1000 | 2ml/dose/day |
The induction phase was undertaken under medical supervision to monitor for any serious adverse events or life threatening anaphylactic reactions. The standardised allergen extract solutions were administered as sublingual drops and patients were instructed to keep the drops under the tongue for 2min before swallowing. As per the dosing regimen which is summarised in Table 1, patients received the extracts at successive doses of 0.1, 0.3, 0.6, 1.0 and 2.0ml of 1000mcg/ml concentration solution (100, 300, 600, 1000 and 2000 Allergoid units respectively) with 5min interval between each dose, for a total duration of 20min, to reach the maximum dose of 2ml (2000AU) or up to the tolerable dose.
Blood pressure and pulse rate were measured before and after each dose. All adverse reactions occurring at each dose level (type of reaction, severity according to the classification of the European Academy of Allergology and Clinical Immunology,18 whether the reaction was local oral, gastrointestinal or systemic, time to onset after administration, reaction duration, measures taken, and relationship to the study drug) were recorded. In order to capture late reactions, patients were kept under observation for 2h after the last dose of the ultra-rush regimen.
Patients were given symptom diary and treatment diary and were instructed to enter the nature and frequency of symptoms and the rescue medications taken during the study period. Patient symptoms and treatment medications were coded and the patients were asked to enter on a daily basis. The treatment medications used were antihistamines 10mg Cetirizine, 180mg Fenofexadine & 25mg Hydroxyzine and Mometasone & Loteprenol nasal sprays.
After the induction phase, patients were advised to take the maintenance dose of 2ml (or tolerable dose) daily in the morning, on an empty stomach. Patients were advised to report any adverse reactions which occurred during the maintenance phase. Subsequently, these patients were followed up for a period of 8 months to assess the clinical efficacy of the ultra-rush sublingual immunotherapy.
Statistical analysisDescriptive statistics such as mean, standard deviation, frequency and percentage were used to describe nominal and ordinal data such as socio-demographic profile, allergen profile and adverse events. Inferential statistics such as Friedman test was used to assess the clinical efficacy of ultra-rush sublingual immunotherapy.
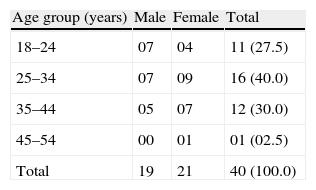
ResultsSocio-demographic profile of allergic rhinitis patientsOut of 40 patients, 16 (40%) patients were in the age group of 25–34 years; followed by 12 (30%) in the age group of 35–44 years; 11 (27.5%) in the age group of 18–24 years and only one (2.5%) patient was in the age group above 45 years. Twenty-one (52.5%) patients were females and the remaining 19 (47.5%) were males. The age of the youngest and the oldest patient was 18 years and 53 years, respectively. The mean age of the patients was 30.38±8.00 years. The mean age of male and female patients was 28.68±8.11 years and 31.90±7.90 years, respectively (Table 2).
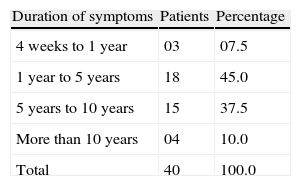
Profile of allergic rhinitis patients according to their history and clinical examinationOut of 40 allergic rhinitis patients, 33 (82.5%) were suffering from allergic rhinitis for a duration of 1–10 years. The mean disease duration was 6.65±5.47 years [range 0.5–30years] (Table 3). Thirty (75%) patients had a history of aggravation of symptoms in the early morning; 34 (85%) patients had a history of aggravation of symptoms on exposure to dust.
Out of 40 patients, 19 (47.5%) patients had family history of atopy. Among them, nine (47.37) patients each had family history of atopy in their maternal and paternal side, and six patients had family history of atopy in their siblings.
According to ARIA guidelines, 26 (65%) were suffering from mild persistent allergic rhinitis and the remaining 14 (35%) were suffering from moderate to severe persistent allergic rhinitis.
Profile of allergen positivity among allergic rhinitis patientsOut of 40 patients recruited for ultra-rush sublingual immunotherapy, 36 (90%) were given immunotherapy for house dust mites (Dermatophagoides farinae and Dermatophagoides pteronyssinus) and the remaining four (10%) patients were given immunotherapy for one or more pollens such as Amaranthus spinosus, Chenopodium album, Cassia siamea, Prosopis juliflora, Peltophorum pterocarpum and Ricinus communis. The details of allergen specific immunotherapy given to each individual have been shown in Table 4.
Distribution of allergic rhinitis patients according to allergen positivity.
| S. no. | Code | Allergen sensitivity |
| 1 | RAK | Prosopis juliflora ++++ |
| 2 | KAR | D. farina ++, D. pteronyssinus +++ |
| 3 | ANR | Amaranthus spinosus +++, Chenopodium album +++, Cassia siamea +++, Ricinus communis +++ |
| 4 | VIB | D. farinae ++, D. pteronyssinus ++, Crab ++ |
| 5 | DIR | D. farinae ++, D. pteronyssinus ++ |
| 6 | SID | D. farinae ++, D. pteronyssinus ++ |
| 7 | HES | D. farinae ++, D. pteronyssinus ++ |
| 8 | RAN | D. farinae +++, Hay dust ++ |
| 9 | MAK | D. farinae +++ |
| 10 | JAG | D. farinae ++, D. pteronyssinus ++ |
| 11 | RAM | House dust ++ |
| 12 | BHK | D. farinae ++, D. pteronyssinus ++ |
| 13 | MAH | D. farinae +++, D. pteronyssinus +++, Egg (white) +++ |
| 14 | HIT | Prosopis juliflora ++++, Peltophorum pterocarpum ++++, Ricinus communis ++++, Cassia siamea ++, Watermelon ++ |
| 15 | SON | D. farinae ++, D. pteronyssinus ++, Prawn ++++, Crab +++ |
| 16 | GOW | D. farinae ++++, D. pteronyssinus ++++, Prawn +++ |
| 17 | SHI | D. farinae ++, D. pteronyssinus ++ |
| 18 | MLA | D. farinae +++, D. pteronyssinus +++ |
| 19 | PAL | D. farinae +++, D. pteronyssinus +++, Rice weevil ++, Crab +++, Dhania ++ |
| 20 | ANJ | D. farinae ++, D. pteronyssinus ++ |
| 21 | UDK | D. farinae ++++, D. pteronyssinus ++++, Kabool gram ++ |
| 22 | SYE | D. farinae +++, D. pteronyssinus +++, Crab +++ |
| 23 | NAR | D. farinae +++, D. pteronyssinus +++, Almond ++, Crab ++ |
| 24 | DIV | D. farinae ++++, D. pteronyssinus ++++, Almond +++ |
| 25 | VEM | D. farinae +++, D. Pteronyssinus +++, Potato ++ |
| 26 | POO | D. farinae ++++, D. pteronyssinus ++++, Ginger ++ |
| 27 | LOM | D. farinae +++, D. pteronyssinus ++++ |
| 28 | VIL | D. farinae ++, D. pteronyssinus ++, Prawn ++++, Moth ++ |
| 29 | SUD | D. farinae +++, D. pteronyssinus ++, Crab +++ |
| 30 | KIK | D. farinae ++, Prawn +++ |
| 31 | SRE | D. farinae +++, D. pteronyssinus +++ |
| 32 | SEK | D. farinae +++, D. pteronyssinus +++, Rice weevil ++, Crab ++ |
| 33 | MTH | D. farinae ++++, D. pteronyssinus ++++ |
| 34 | SAD | D. farinae +++, D. pteronyssinus +++ |
| 35 | SIN | D. farinae +++, D. pteronyssinus ++++ |
| 36 | MSH | D. farinae ++++, D. pteronyssinus ++++ |
| 37 | BHS | D. farinae +++, D. pteronyssinus +++ |
| 38 | VIA | D. farinae ++++, D. pteronyssinus ++++ |
| 39 | DEE | D. farinae ++++, D. pteronyssinus ++++, |
| 40 | DIA | Chenopodium album +++, Peltophorum pterocarpum +++, Prosopis juliflora +++ |
Patients were advised to avoid sensitive food allergens.
In the present study, all patients tolerated the complete dosage of ultra-rush SLIT regimen. No patient discontinued the treatment due to adverse events, and no serious adverse events or life threatening anaphylactic reactions were reported. All these patients were observed for local oral, local gastrointestinal and systemic adverse reactions and graded as per their severity.
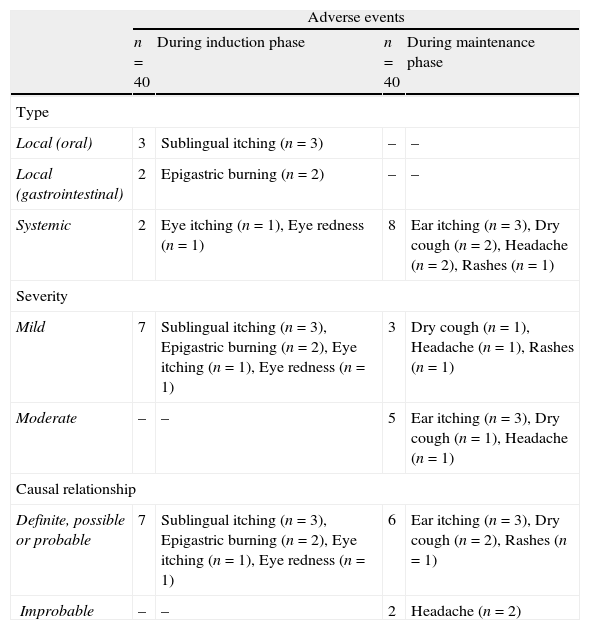
Safety results for the induction phase at hospitalOut of 40 patients who had undergone ultra-rush sublingual immunotherapy, only six patients developed seven adverse reactions. Thirty-four (85%) patients did not experience any adverse reaction during the induction phase. Out of six patients who developed seven adverse reactions, two patients reported local oral reactions in the form of sublingual itching. One patient reported local gastrointestinal reaction in the form of epigastric burning and two patients developed systemic reactions: one with eye itching and one with eye redness. One patient had both sublingual itching and epigastric burning.
Regarding severity, all the seven immediate adverse reactions were mild in severity and they were resolved without any medications within 30min of observation. The ultra-rush regimen was not modified in any patient because of adverse reactions (Tables 5 and 6).
Type and duration of adverse events reported during the study.
| Adverse events | ||||
| n=40 | During induction phase | n=40 | During maintenance phase | |
| Type | ||||
| Local (oral) | 3 | Sublingual itching (n=3) | – | – |
| Local (gastrointestinal) | 2 | Epigastric burning (n=2) | – | – |
| Systemic | 2 | Eye itching (n=1), Eye redness (n=1) | 8 | Ear itching (n=3), Dry cough (n=2), Headache (n=2), Rashes (n=1) |
| Severity | ||||
| Mild | 7 | Sublingual itching (n=3), Epigastric burning (n=2), Eye itching (n=1), Eye redness (n=1) | 3 | Dry cough (n=1), Headache (n=1), Rashes (n=1) |
| Moderate | – | – | 5 | Ear itching (n=3), Dry cough (n=1), Headache (n=1) |
| Causal relationship | ||||
| Definite, possible or probable | 7 | Sublingual itching (n=3), Epigastric burning (n=2), Eye itching (n=1), Eye redness (n=1) | 6 | Ear itching (n=3), Dry cough (n=2), Rashes (n=1) |
| Improbable | – | – | 2 | Headache (n=2) |
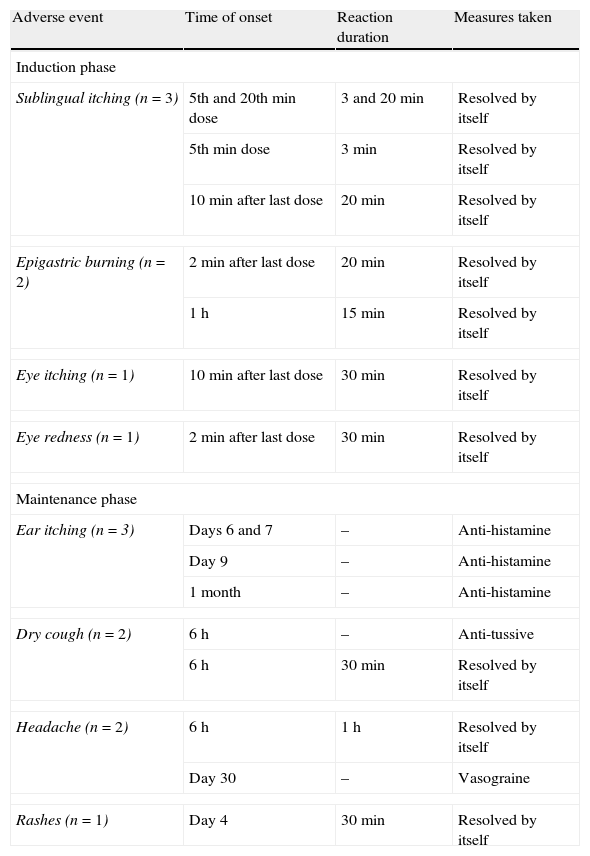
Duration of adverse events reported during the study and measures taken.
| Adverse event | Time of onset | Reaction duration | Measures taken |
| Induction phase | |||
| Sublingual itching (n=3) | 5th and 20th min dose | 3 and 20min | Resolved by itself |
| 5th min dose | 3min | Resolved by itself | |
| 10min after last dose | 20min | Resolved by itself | |
| Epigastric burning (n=2) | 2min after last dose | 20min | Resolved by itself |
| 1h | 15min | Resolved by itself | |
| Eye itching (n=1) | 10min after last dose | 30min | Resolved by itself |
| Eye redness (n=1) | 2min after last dose | 30min | Resolved by itself |
| Maintenance phase | |||
| Ear itching (n=3) | Days 6 and 7 | – | Anti-histamine |
| Day 9 | – | Anti-histamine | |
| 1 month | – | Anti-histamine | |
| Dry cough (n=2) | 6h | – | Anti-tussive |
| 6h | 30min | Resolved by itself | |
| Headache (n=2) | 6h | 1h | Resolved by itself |
| Day 30 | – | Vasograine | |
| Rashes (n=1) | Day 4 | 30min | Resolved by itself |
During 4 months of maintenance phase, eight delayed adverse reactions were reported in seven patients. All eight were systemic reactions: three with ear itching, two with dry cough, two with headache and one with rashes over forearm. None of them reported any local oral or local gastrointestinal reaction.
Regarding severity among those who had delayed systemic adverse reactions, three reactions (dry cough, head ache, rash, in one each) were mild in severity and they were resolved without any medications within 1h. The five remaining reactions, i.e., ear itching in three, dry cough in one and headache in one were moderate in severity and they were resolved with symptomatic oral medications (Tables 5 and 6).
Assessment of clinical efficacy of ultra-rush SLITThe patients were given a symptom diary and treatment diary, instructed to enter the nature and frequency of symptoms and rescue medications taken, and followed up for 12 months to assess the clinical efficacy of ultra-rush SLIT (4 months of maintenance period and 8 months of follow-up period).
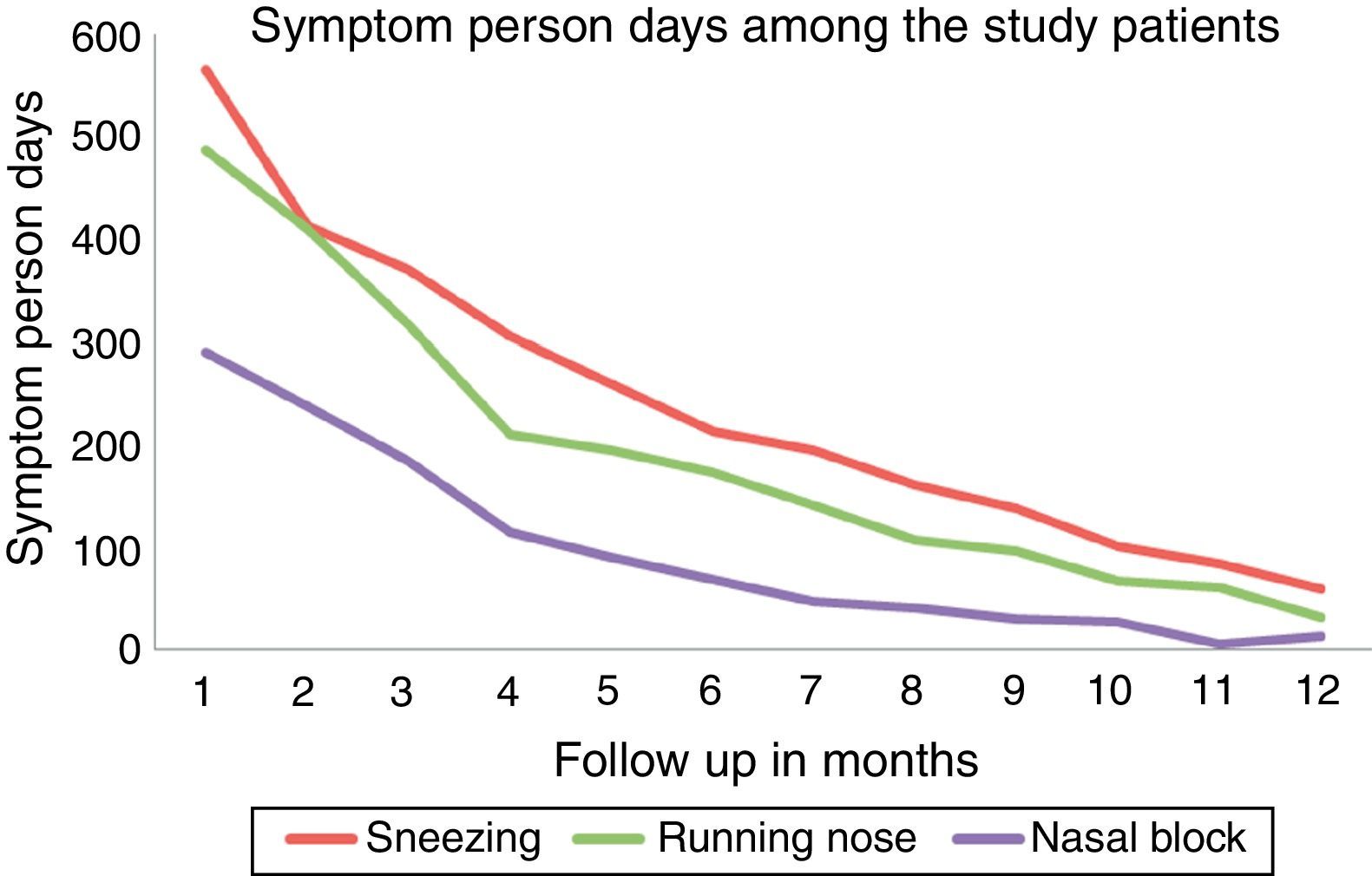
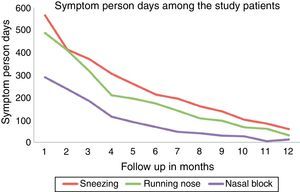
Among the allergic rhinitis patients who received ultra-rush SLIT, it was observed that the number of symptom person-days (number of days the patient had symptoms) decreased gradually over a period of 12 months follow up showing good clinical efficacy which was statistically significant [Friedman test, Fr=198.010, P=0.001] (Fig. 1).
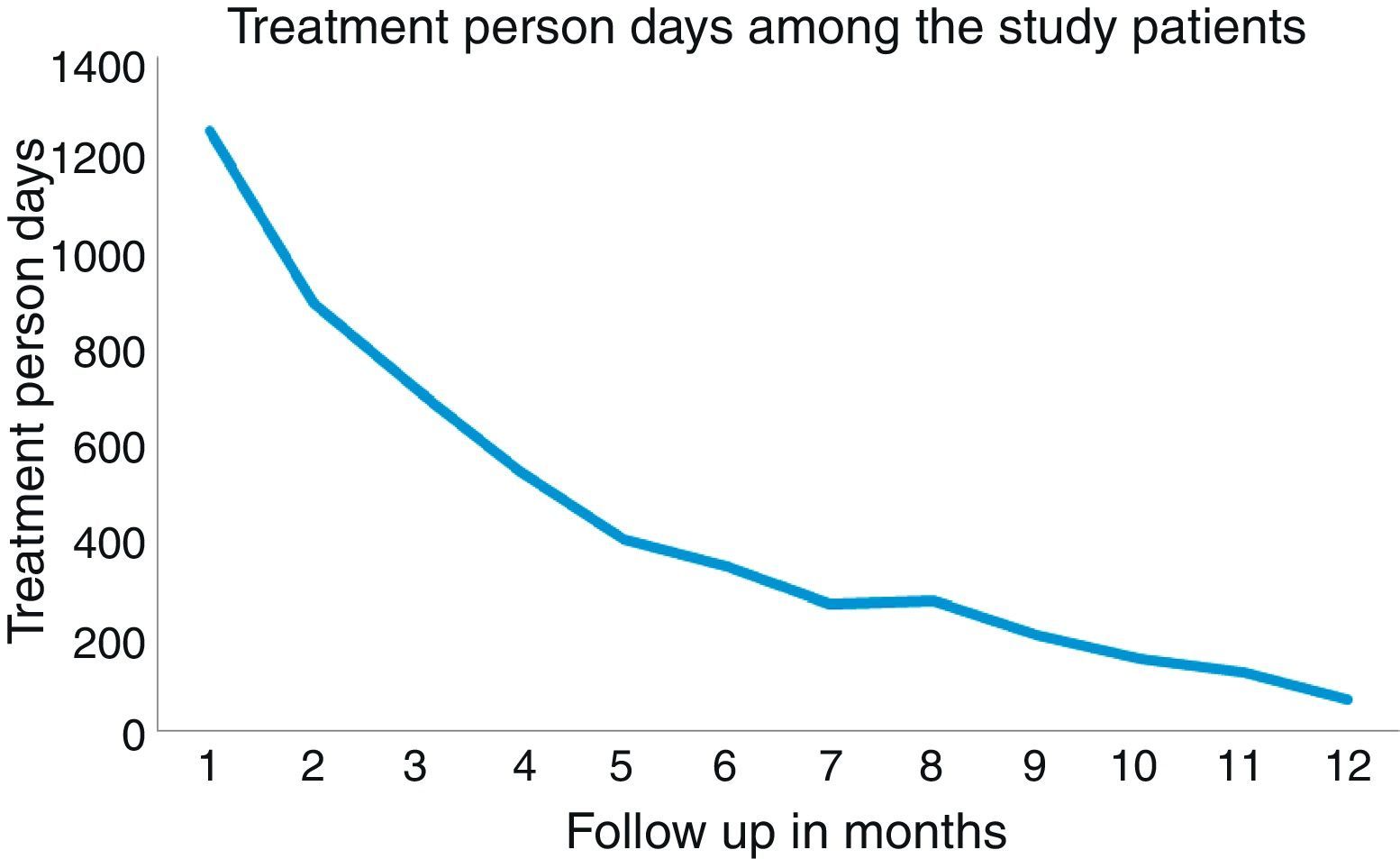
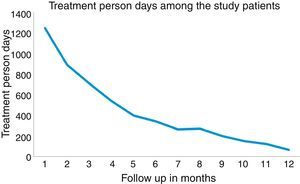
Similarly, the number of treatment person-days (number of days on which the patient was on rescue medications) decreased gradually over a period of 12 months follow up, showing good clinical efficacy which was statistically significant [Friedman test, Fr=230.924, P=0.001] (Fig. 2).
DiscussionThe clinicians began to seek new regimens agreeable to patients encouraged by the good tolerance of sublingual therapies. The use of ultra-rush sublingual immunotherapy regimens – where the induction phase is compressed into less than a couple of hours – represents a compromise between conventional updosing and its complete elimination. The ultra-rush SLIT regimens are administered under strict monitoring of an allergist, in a medical centre with the necessary resources to deal with any life-threatening events such as anaphylactic reactions. The maintenance doses are then administered in the patient's home without any medical supervision (it is of utmost importance that this part of the regimen is well-tolerated).2
In the present study, the mean age of the patients was 30.38±8.00years (range 18–53years). The mean age of male and female patients was 28.68±8.11 years and 31.90±7.90 years respectively. This observation differs from the study conducted by Roger et al.2 where the mean age of the patients was 20.4 years (range 4–64 years). It is in accordance with the study conducted by Gammeri et al.,9 where among 105 patients there were 28 children (20 males, mean age 13.3±2.1 years) and 77 adults (29 males, mean age 34.7±9.9 years). In the study by Seidenberg et al.,4 193 children and adolescents included 66 girls (34%) and 127 boys (66%), aged 5–17 years (mean age, 10.3 years).
The mean disease duration was 6.65±5.47 years [range 0.5–30 years]. This was longer than in the study conducted by Roger et al.,2 where the mean disease duration was 4.84 years.
In the present study, 19 (47.5%) patients had a family history of atopy, which was similar to the study conducted by Roger et al.2 where the family history of atopy was present in 107 (49.1%) patients.
According to ARIA guidelines, 26 (65%) were suffering from mild persistent allergic rhinitis and the remaining 14 (35%) were suffering from moderate-severe persistent allergic rhinitis. Pharmacotherapy was preferred in mild intermittent and moderate-severe intermittent allergic rhinitis and hence these patients were not included in the present study. Whereas Roger et al.2 studied on 218 allergic rhinitis patients. Gammeri et al.9 studied on 64 intermittent/persistent allergic rhinitis patients and 41 mild/persistent asthma patients. Seidenberg et al.4 observed 182 allergic rhinitis patients in their study.
In the present study, the patients were sensitive to house dust mites (Dermatophagoides farinae and Dermatophagoides pteronyssinus) and pollens such as Amaranthus spinosus, Chenopodium album, Cassia siamea, Prosopis juliflora, Peltophorum pterocarpum and Ricinus communis. In contrast to the study conducted by Roger et al.,2 patients who were sensitive to house dust mites were included. In Gammeri et al.9 20min ultra-rush SLIT study, patients who were sensitive to house dust mite, Parietaria and Timothy grass were included. Seidenberg et al.4 carried out an observational study on patients who were sensitive to grass pollens (cocksfoot, meadow grass, rye grass, sweet vernal grass, timothy) or tree pollens (birch, alder, hazel).
In the present study, all the patients tolerated the complete dosage of ultra-rush SLIT regimen. No patient discontinued the treatment due to adverse events, and no serious adverse events or life threatening anaphylactic reactions were reported, which was similar to the Gammeri et al. study9 where all patients tolerated the treatment very well. The results of the Seidenberg et al. study4 demonstrated a good to very good tolerability of ultra-rush titration. The findings of the present study differ from the Roger et al.2 study where five patients had to modify ultra-rush regimen due to their adverse events.
In the present study, all the adverse reactions in the induction phase were mild in severity and they were resolved without any medications within 30min of observation. This finding was similar to the study conducted by Roger et al.2 where they reported 32 adverse reactions in 27 (12.4%) patients during induction phase. Seven events were local gastrointestinal reactions, 17 were local oral reactions and eight were systemic. All adverse reactions were mild or moderate and no serious adverse event or life threatening anaphylactic reactions were reported. Gammeri et al.9 in their study reported that only one patient out of 105 (0.9%) had a mild local symptom that occurred 30min after the last initial dose and spontaneously disappeared within 1h. The present study's findings differ from the study conducted by Seidenberg et al.,4 where during ultra-rush titration, 60 patients (31%) reported 117 predominantly mild and local adverse events, which were resolved within 150min. This justifies the 2-h observation period of patients following the induction phase.
In the present study, eight delayed adverse reactions were reported in seven patients in the maintenance phase. Three reactions were mild in severity and they were resolved without any medications within 1h. The remaining five reactions were moderate in severity and they were resolved with symptomatic oral medications. This was similar to the study conducted by Roger et al.2 where 25 (12%) experienced mild or moderate local reactions during the maintenance phase. The present study's findings differ from the study conducted by Seidenberg et al.,4 where 562 adverse events were reported during the maintenance phase. The most frequent local events were oral pruritus, burning sensation, lip or tongue swelling, and gastrointestinal symptoms, and the most frequent systemic events were rhinoconjunctivitis and asthma.
In the present study, it was observed that both the number of symptom person-days and also the number of treatment person-days decreased gradually over a period of 12 months follow up (P=0.001). This was similar to the study conducted by Vervloet et al.5 where there was a marked and significant (P<0.03) decrease of the medication score (about 50%) and nasal steroid consumption (about 75%) in the active treatment group. These findings were similar to other studies.7,19
The limitation of this study is that the long-term benefits of ultra-rush sublingual immunotherapy in the heterogeneous study subjects could not be studied because of time constraints. Although a randomised control study would be the appropriate study design, it was not done due to feasibility and ethical constraints.
To conclude, ultra-rush sublingual immunotherapy regimen has excellent safety, tolerability and clinical efficacy among patients suffering from allergic rhinitis.
Hence ultra-rush sublingual immunotherapy can be recommended as an alternative to conventional sublingual immunotherapy. There is a need for further studies like randomised controlled trials involving a larger sample size and with a longer follow-up period.
Ethical disclosuresProtection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in the Study and the authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors have no conflict of interest to declare.
A part of the data was presented in IAACON 2011 biennial conference, Bangalore, India on 14.8.2011 and was awarded the best paper in the same conference. Dr. Ruby Pawankar, Dr. Rossenwaller, Dr. Walter Canonica also attended the conference.