Conflicting results have been reported, mostly in developed countries, on the relationship between exposure to traffic and allergic diseases. This study aims to examine the impact of truck traffic on asthma, rhinitis and eczema in early adolescence in Skopje, the capital of the Republic of Macedonia, as a developing country with a lower middle rate of high truck traffic exposure and low prevalence rates of allergic diseases.
MethodsSelf-reported data was used, obtained through the International Study of Asthma and Allergies in Childhood Phase 3 written questionnaires, from 3026 adolescents aged 13–14 years from Skopje. Truck traffic density on the street of residence on weekdays was correlated to current and ever-diagnosed asthma, rhinitis and eczema by odds ratios (OR, 95% CI) in binary logistic regression, with and without adjustments for potential confounding factors separately and for their joint effect.
ResultsA positive association of truck traffic density appeared to be limited to current dry night cough (aOR: 1.63; 1.07–2.47; aOR: 2.17; 1.40–3.35; and aOR: 2.33; 1.43–3.79 for truck traffic seldom, frequently through the day, and almost the whole day, respectively) with an exposure–response relationship and to current wheeze only for truck traffic almost the whole day (aOR: 1.87; 1.02–3.42).
ConclusionThe findings suggest an aggravating effect of truck traffic on current asthma symptoms, but not on asthma, allergic rhinitis and eczema diagnoses. It seems that it probably has an impact as a direct respiratory irritant in early adolescence.
The association of traffic air pollution with allergic diseases has attracted a great deal of scientific interest in recent years. A number of earlier and more recent studies in children in developed countries, using different instruments for traffic air pollution assessment, have documented inconsistent results.
Some studies have described positive associations of traffic-related air pollutants or proximity to main roads with wheeze and asthma diagnosis1 and hay fever,2 but not with atopic eczema2; contrary to other studies observing positive association also with the latter.3 Self-reported truck traffic density has also been found to increase the risk of allergic diseases.4 However, other studies have not confirmed such a relationship.5,6
A limited number of studies have focussed on the association between traffic air pollution and night cough, one of which has reported a positive relationship7 while others8,9 have failed to find such an association.
Even though the mechanisms are not fully understood, oxidative stress and inflammation have been suggested as the major underlying mechanisms behind many of the toxic reactions induced by air pollutants.10,11
Compared to worldwide prevalence rates of asthma, allergic rhinitis and eczema,12–14 the Republic of Macedonia appears to have a moderately low prevalence of current wheeze and low prevalence rates of current allergic rhinitis and eczema symptoms. According to the International Study of Asthma and Allergies in Childhood (ISAAC) Phase 3 global analysis, the Northern-Eastern European region in which Macedonia is located has a lower middle rate of high truck traffic density.4 On the other hand, dietary antioxidants intake has been documented to be high in Macedonia,15 which may be explained by the geographical area in which the country is situated and its climate.
The present study aims to explore the association between self-reported truck traffic density as a proxy for exposure to traffic-related air pollutants, and asthma and allergic rhinitis and eczema in young adolescents in Skopje, the capital of the Republic of Macedonia, as a developing country with low to medium truck traffic exposure and low prevalence rates of allergic diseases.
Subjects and methodsThe study was cross-sectional and was conducted during 2001–2004 in the capital of the Republic of Macedonia, Skopje, as part of the ISAAC Phase 3. Selection of participants and data collection were performed strictly in accordance with the ISAAC methodology.16,17 The standardised ISAAC Phase 3 written questionnaires on asthma, rhinitis, eczema, and environmental risk factors were self-completed by 3026 adolescents 13–14 years old from 17 randomly-selected state schools. No additional questions were included in the original form of the questionnaires. After informed consent was obtained from parents, the data collection was carried out during the first four months of 2002. The response-rate was 90.9%.
The health outcomes of interest in the study were current wheeze, current exercise-induced wheeze, current dry night cough and ever-diagnosed asthma; current nose symptoms, current nose and eye symptoms and ever-diagnosed hay fever; current symptoms of eczema and ever-diagnosed eczema, as these were previously defined.12–16
Truck traffic density on the street of residence on weekdays by different category (seldom, frequently through the day, and almost the whole day, versus a ‘never’-category used as a reference) was assessed separately in relation to current and ever-diagnosed asthma, allergic rhinitis and eczema prevalence rates in order to explore the existence of an exposure–response relationship. Gender and the following environmental factors were used as potential confounders in adjusted analyses. Current fruit and cereals intake, as an indicator of external antioxidant intake, was classified as frequent (≥3 times a week) versus infrequent (never/occasionally and 1–2 times a week). Current use of Paracetamol classified as frequent (at least once monthly) versus infrequent (at least once yearly and never), and gas and wood used for cooking and wood/coal/oil used for heating at home were all employed as external oxidants. The mother's educational level (tertiary vs. secondary/primary) and existence of siblings (presence vs. absence) were both used as familiar socioeconomic status indicators. Additionally, passive smoking exposure at home was included amongst the confounding factors as a source of inhaled oxidants as well as a socioeconomic status indicator.
Of the total number of respondents, 1568 (51.8%) were boys and 1458 (48.2%) were girls. Their mean average age was 13.45 years (SD 0.50).
Ethical approvalThe conducting of the ISAAC Phase 3 in The Republic of Macedonia has been approved by The Ethics Committee at the Medical Faculty and The Ministry of Education and Science, Skopje, the Republic of Macedonia.
Statistical analysesMissing or “any other” responses were part of the denominator for the calculation of asthma, allergic rhinitis and eczema prevalence figures, which was not the case with respect to truck traffic density and potential confounding factors.18
The associations of truck traffic density—both unadjusted and adjusted for the potential confounders—with current and ever-diagnosed asthma and allergic rhinitis and eczema were analysed by odds ratios with 95% confidence intervals (OR, 95% CI) in logistic regression in SPSS 11.0 for Windows. Additionally, the interaction of truck traffic density with each confounding factor one by one was analysed for current exercise-induced wheeze and current itchy rash. The referent category used was that of ‘never’ having truck traffic on a residential street.
A resulting p-value of <0.05 was considered significant.
ResultsThe established overall prevalence rates of truck traffic density on residential streets and current and ever-diagnosed asthma, rhinitis and eczema in respondents, as well the prevalence rates of the same outcomes by truck traffic exposure category, are shown in Table 1. The most frequently reported case (54.9%) was of trucks passing on residential streets seldom on weekdays, while 10% reported truck traffic almost the whole day. Almost equal prevalence rates of truck traffic density exposure in girls compared to boys were observed (54.7% vs. 55.0%, 27.0% vs. 24.9%, and 9.9% vs. 10.0% for truck traffic seldom, frequently during the day, and almost the whole day, respectively).
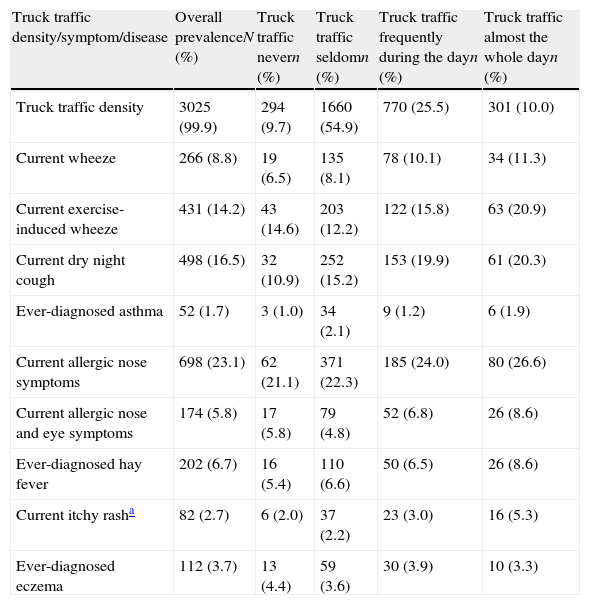
Overall prevalence rates of truck traffic density and asthma, allergic rhinitis, eczema-related symptoms and diagnoses and their prevalence rates by category of traffic density (written questionnaires) in 3026 children aged 13–14 years in Skopje, The Republic of Macedonia, 2001/2004.
| Truck traffic density/symptom/disease | Overall prevalenceN (%) | Truck traffic nevern (%) | Truck traffic seldomn (%) | Truck traffic frequently during the dayn (%) | Truck traffic almost the whole dayn (%) |
| Truck traffic density | 3025 (99.9) | 294 (9.7) | 1660 (54.9) | 770 (25.5) | 301 (10.0) |
| Current wheeze | 266 (8.8) | 19 (6.5) | 135 (8.1) | 78 (10.1) | 34 (11.3) |
| Current exercise-induced wheeze | 431 (14.2) | 43 (14.6) | 203 (12.2) | 122 (15.8) | 63 (20.9) |
| Current dry night cough | 498 (16.5) | 32 (10.9) | 252 (15.2) | 153 (19.9) | 61 (20.3) |
| Ever-diagnosed asthma | 52 (1.7) | 3 (1.0) | 34 (2.1) | 9 (1.2) | 6 (1.9) |
| Current allergic nose symptoms | 698 (23.1) | 62 (21.1) | 371 (22.3) | 185 (24.0) | 80 (26.6) |
| Current allergic nose and eye symptoms | 174 (5.8) | 17 (5.8) | 79 (4.8) | 52 (6.8) | 26 (8.6) |
| Ever-diagnosed hay fever | 202 (6.7) | 16 (5.4) | 110 (6.6) | 50 (6.5) | 26 (8.6) |
| Current itchy rasha | 82 (2.7) | 6 (2.0) | 37 (2.2) | 23 (3.0) | 16 (5.3) |
| Ever-diagnosed eczema | 112 (3.7) | 13 (4.4) | 59 (3.6) | 30 (3.9) | 10 (3.3) |
Current=in the last 12 months.
Except in the case of ever-diagnosed eczema, for all other outcomes the prevalence rates were significantly higher in respondents exposed to truck traffic almost the whole day compared to those not exposed at all. There were positive and graded associations between truck traffic density and current wheeze, current dry night cough, current allergic nose symptoms and current itchy rash by higher unadjusted prevalences of symptoms with higher frequency of truck traffic (Table 1).
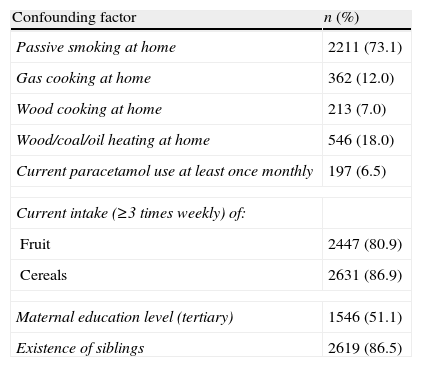
The majority of the respondents reported frequent current intake of fruit (80.9%) and cereals (86.9%) and the existence of siblings (86.5%) (Table 2).
Prevalence rates of confounding factors (written questionnaire) in 3026 children aged 13–14 years in Skopje, The Republic of Macedonia, 2001/2004.
| Confounding factor | n (%) |
| Passive smoking at home | 2211 (73.1) |
| Gas cooking at home | 362 (12.0) |
| Wood cooking at home | 213 (7.0) |
| Wood/coal/oil heating at home | 546 (18.0) |
| Current paracetamol use at least once monthly | 197 (6.5) |
| Current intake (≥3 times weekly) of: | |
| Fruit | 2447 (80.9) |
| Cereals | 2631 (86.9) |
| Maternal education level (tertiary) | 1546 (51.1) |
| Existence of siblings | 2619 (86.5) |
Current=in the last 12 months.
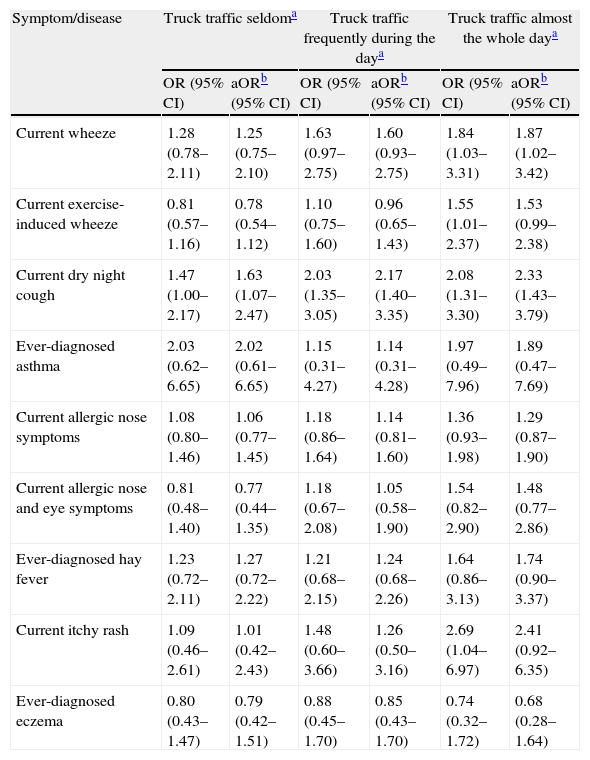
Current dry night cough was found to be positively associated with all three categories of truck traffic density, both before and after adjustment for potential confounding factors, in an exposure–response relationship. Current wheeze was positively associated with truck traffic only if it was almost the whole day, in the unadjusted as well the adjusted analysis. In the unadjusted analyses, significant positive associations were established between truck traffic almost the whole day and current exercise-induced wheeze and current itchy rash but after adjusting for all confounders to assess their joint effect these associations were somewhat reduced and no longer statistically significant (p=0.045 and p=0.058; p=0.042 and p=0.074, respectively) (Table 3). To explore which confounder had effect modification on adjusted ORs, the interaction of each of them was analysed one by one with traffic exposure for latter outcomes. Effect modification was observed only for gender in relation to current exercise-induced wheeze (p=0.066) and for gender and Paracetamol intake in relation to current itchy rash (p=0.053 and p=0.062, respectively). Afterwards, analyses of both the unadjusted and adjusted for all confounders truck traffic exposure and current exercise-induced wheeze and itchy rash as outcomes were performed in boys and girls separately, but no significant associations were observed at all (data not shown).
Relationship of truck traffic density with current and ever-diagnosed asthma, allergic rhinitis and eczema in 3026 children aged 13–14 years in Skopje, The Republic of Macedonia, 2001/2004.
| Symptom/disease | Truck traffic seldoma | Truck traffic frequently during the daya | Truck traffic almost the whole daya | |||
| OR (95% CI) | aORb (95% CI) | OR (95% CI) | aORb (95% CI) | OR (95% CI) | aORb (95% CI) | |
| Current wheeze | 1.28 (0.78–2.11) | 1.25 (0.75–2.10) | 1.63 (0.97–2.75) | 1.60 (0.93–2.75) | 1.84 (1.03–3.31) | 1.87 (1.02–3.42) |
| Current exercise-induced wheeze | 0.81 (0.57–1.16) | 0.78 (0.54–1.12) | 1.10 (0.75–1.60) | 0.96 (0.65–1.43) | 1.55 (1.01–2.37) | 1.53 (0.99–2.38) |
| Current dry night cough | 1.47 (1.00–2.17) | 1.63 (1.07–2.47) | 2.03 (1.35–3.05) | 2.17 (1.40–3.35) | 2.08 (1.31–3.30) | 2.33 (1.43–3.79) |
| Ever-diagnosed asthma | 2.03 (0.62–6.65) | 2.02 (0.61–6.65) | 1.15 (0.31–4.27) | 1.14 (0.31–4.28) | 1.97 (0.49–7.96) | 1.89 (0.47–7.69) |
| Current allergic nose symptoms | 1.08 (0.80–1.46) | 1.06 (0.77–1.45) | 1.18 (0.86–1.64) | 1.14 (0.81–1.60) | 1.36 (0.93–1.98) | 1.29 (0.87–1.90) |
| Current allergic nose and eye symptoms | 0.81 (0.48–1.40) | 0.77 (0.44–1.35) | 1.18 (0.67–2.08) | 1.05 (0.58–1.90) | 1.54 (0.82–2.90) | 1.48 (0.77–2.86) |
| Ever-diagnosed hay fever | 1.23 (0.72–2.11) | 1.27 (0.72–2.22) | 1.21 (0.68–2.15) | 1.24 (0.68–2.26) | 1.64 (0.86–3.13) | 1.74 (0.90–3.37) |
| Current itchy rash | 1.09 (0.46–2.61) | 1.01 (0.42–2.43) | 1.48 (0.60–3.66) | 1.26 (0.50–3.16) | 2.69 (1.04–6.97) | 2.41 (0.92–6.35) |
| Ever-diagnosed eczema | 0.80 (0.43–1.47) | 0.79 (0.42–1.51) | 0.88 (0.45–1.70) | 0.85 (0.43–1.70) | 0.74 (0.32–1.72) | 0.68 (0.28–1.64) |
OR=Odds ratio; aOR=adjusted OR; CI=confidence interval; current=in the last 12 months.
As the established prevalence of high truck traffic density on the street of residence in our respondents was 10.0%, which is a borderline rate between the low and middle ones for high truck traffic density estimated worldwide4 and the prevalence rates of the investigated diseases were low,12–14 a strong positive relationship could be assumed between both, which was not the case.
The results of the present study showed a clear positive association only between current night dry cough, apart from chest infection, and truck traffic regardless of its density, with an exposure–response relationship. The association between this asthma symptom and air pollution exposure is still unclear because of the inconsistent results in few published studies, especially related to the age of the children exposed. Our finding is in line with the results from a German survey also based on self-reported data obtained through the ISAAC Phase 3 questionnaires in 13–14-year-old children, where the same results have not been found in 6–7-year-old children.7 Additionally, Gehring et al.19 have observed that dry night cough was positively associated with long-term exposure to traffic-related air pollutants such as particulate matter (PM2.5), PM2.5 absorbance and NO2 exposure in infants, but not in children of two years of age. A prospective birth cohort study has documented an increased risk of recurrent dry night cough and wheeze in young children with familial atopy who were highly exposed to traffic exhaust fumes, assessed through objective measures.10 Brauer et al.,9 contrary to the results of our own and the abovementioned studies, have reported a weak positive association of dry night cough with soot, NO2 and PM2.5 only before adjustment for confounders in four-year-old children.
In the present study, current wheeze was positively associated only with high truck traffic density. No significant associations were observed in relation to other outcomes. ISAAC Phase 1 and ISAAC Phase 3 studies, both conducted in Germany, have provided evidence that self-reported truck traffic density was positively related to current wheeze and rhinitis symptoms, but not to asthma or hay fever diagnoses in young adolescents.7,20 ISAAC Phase 3 global analysis showed a positive association between self-reported truck traffic density and the prevalence rates of current asthma, rhinoconjunctivitis and eczema symptoms, and ever-diagnosed asthma in 13–14-year-olds and 6–7-year-olds with an exposure–response relationship in many locations of the world.4 However, compared to these ISAAC studies, other confounders, including dietary antioxidant intake, were used in our study. Information about the active smoking habits of the respondents, and familial atopy which can also alter our results, were not considered since we have not modified the ISAAC Phase 3 questionnaires for 13–14-year-olds.
Other studies have used more objective measures for traffic-related air pollution and have investigated different allergic outcomes, observing different findings. In most earlier studies, eczema had not been looked at because of the belief that eczema could not be influenced by air pollution. However, as enhanced allergic sensitisation to inhalant21 and to food allergens9 by truck traffic air pollutants in prospective birth cohort studies has already been documented, the assumed possibility of systemic immune modulation by diesel particles seems reasonable. Morgenstern et al.,3 assessing the influence of long-term exposure to traffic-related air pollutants in young children, have documented a higher risk of eczema by NO2 and a higher risk of asthma, hay fever and allergic sensitisation to pollen by particulate matter (PM2.5) absorbance. On the other hand, in a prospective birth cohort study at four years of age, positive associations of traffic air pollutants with wheeze, asthma and sensitisation to food allergens, but not with eczema or sensitisation to inhalant allergens, were observed.9 Kramer et al.22 have documented an increased risk of hay fever by outdoor NO2, contrary to the study performed by Wyler et al.,23 who did not find such an association using traffic counts at the domiciles of subjects. Eczema, in contrast to our own and some other findings,2,24 has been found to be positively associated with traffic air pollution in Taiwanese school children as well.25
However, some studies have documented no association of proximity of home from the nearest main road or of traffic counts or of residential NO2 levels either with current wheeze or diagnosed asthma or eczema symptoms5 or with current and ever-diagnosed asthma, hay fever and eczema.24,26 In contrast, the consistency of the results from cohort studies reviewed by Braback and Forsberg11 indicated that traffic exhaust contributed to the development of respiratory symptoms in healthy children.
Exposure assessment of traffic-related air pollution (ecological designs to public traffic counts, modelled estimations of air pollutant levels, distance of homes to major roads, self or parental-reported traffic density) and applied potential confounders, have varied significantly among different studies, which may explain some of the inconsistent findings.7,11 Indirect factors such as social deprivation, stress related to noise or accident risk associated with busy roads may also influence allergic responses due to traffic-related air pollution.4 Additionally, sensitisation and bronchial hyper-responsiveness (BHR),24 antioxidant intake27 and genetic makeup (polymorphism in genes involved in airway inflammation and oxidative stress)28 have been found to be effect modifiers of the association between traffic exposure and respiratory symptoms and allergy.
The lack of a stronger association between truck traffic exposure and allergic diseases in our study has no ready explanation. This may be due to the protective effect of high dietary antioxidant (fruit and cereals) intake and the high prevalence of siblings or to some other unmeasured confounders in the lifestyle of the young adolescents in our country. No information was available about traffic exposure at places other than home. However, we assume that our 13–14-year-old respondents spent the majority of the time in their neighbourhood as they were obliged by law to attend the nearest primary school. With no information on the duration of living in the present dwelling of our respondents, its possible modification effect could not be assessed, as it was in the study conducted by Duhme et al.20 Janssen et al.24 have observed increased respiratory symptoms near motorways with high truck but not high car traffic counts and this increase was almost entirely restricted to 7–12-year-old children with BHR and/or sensitisation to common allergens. In the capital of our country, car traffic is higher compared to truck traffic exposure and, according to the low prevalence rates of ever-diagnosed asthma and hay fever and eczema, the expected prevalence of adolescents with BHR and/or sensitisation to common allergens would be low as well, which may be another explanation of our findings. Furthermore, we can speculate that higher levels of truck traffic exposure may be needed to reveal notable positive associations with the investigated outcomes. Finally, a genetic variation and an epigenetic modification of certain genes may be associated with individual susceptibility and with the effects of air pollution.
The present study is based on self-reported data and the effect estimate may be influenced by reporting bias. Under-diagnosis of asthma and hay fever and over-diagnosis of eczema may affect the corresponding findings as well. On account of the almost equal gender distribution and prevalence rates of truck traffic density exposure in boys and girls, the initial analyses were conducted on the whole group of respondents with gender included as a confounding factor.
Although it is difficult to discuss aetiological mechanisms in the context of a cross-sectional study based on self-reported data, the documented increased risk only for current night cough and wheeze, with no associations regarding ever-diagnosed allergic diseases in our respondents living near busy streets, is in line with the hypothesis of non-specific irritative rather than allergic inflammatory changes in the airway mucosa caused by traffic air pollution.
In conclusion, the results of the present study provide additional evidence of adverse health effects due to exposure to traffic air pollution. The observed increased risk only for current asthma symptoms in relation to truck traffic exposure in young adolescents supports the hypothesis that components of air pollution act as non-specific respiratory irritants.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans and animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interestThe authors have no conflict of interest to declare.
The authors would like to thank children for their participation, and the principals, psychologists, and teachers for their collaboration in the survey. The Ministry of Education and Science of The Republic of Macedonia provided financial support for the study.