INTRODUCTION
Defense against microbes is mediated by the early reactions of innate immunity and the later response of adaptative immunity. Adaptative immunity is stimulated by exposure to infectious agents, which make the body capable of recognizing and reacting to a larger number of microbial and nonmicrobial substances. Innate or natural immunity consists of cellular and biochemical defense mechanisms that are in place even before infection and poised to respond rapidly to infection. These mechanisms react only to microbes and not to noninfectious substances, and they respond in essentially the same way to repeated infections. The principal components of innate immunity are physical and chemical barriers, phagocytic cells and NK cells, blood proteins - including members of the complement system and other mediators of inflammation, like lactoferrin and Iysozyme.
Lysozyme is a 1-4-β-N acetylmuramidase, which acts on the peptideoglycan layer of Gram positive bacterial wall, causing cell lysis. It's a soluble endoglycosidase present in blood, in externaI fluids as well in the Iysossomal granules of the phagocytes1,2. Lysozyme is also present in human milk throughout lactation in a concentration of 100 μg/ml, and unlike other factors, its concentration increases towards the end of lactation. Lysozyme is produced by monocytes, neutrophils, Paneth cells (in the intestinal tract)3 and salivary glands4. In phylogeny, Iysozyme is highly conserved, being present right through from plants to mammals5.
Primary immunodeficiency disorders are a diverse group of illnesses that, as a result of abnormalities in the immune system, increases susceptibility to infection6. However, little is known about mucosal innate soluble factors that contribut to the host defense of these patients. Synergism between Iysozyme, IgA and the complement system has been reported to occur in microorganism Iysis, but the biological role of Iysozyme is not yet fully known7. In the present work, we investigated the Iysozyme concentrations in saliva from patients with primary immunodeficiencies to verify if Iysozyme might act as a compensatory mechanism for the mucosal protection of these patients.
MATERIAL AND METHODS
Thirty saliva samples were collected from children and adolescents aged between 3 and 13 years and 4 from adults aged between 20 and 33 years at Instituto da Criança of Hospital das Clínicas and Escola Paulista de Medicina, São Paulo, Brazil.
The deficiency diagnosis was: X-linked Agammaglobulinemia (8 cases), Ataxia Telangiectasia Syndrome (5 cases), Common Variable Immunodeficiency (6 cases), Polysaccharide Antibody Deficiency which represent normal serum levels of immunoglobulins and IgG subclasses, good antibody response to proteic antigens and severely impaired antibody response to polysaccharide antigens (4 cases), Hyper-lgM Syndrome (2 cases), Chronic Granulomatous Disease (2 cases), IgG2 Deficiency and Down Syndrome (1 case), Silver-Russel Syndrome with Common Variable Immunodeficiency (1 case), Chédiak Higashi Syndrome (1 case), Selective IgA Deficiency (1 case), Hyper IgE Syndrome (1 case), and Agammaglobulinemia with partial deletion of chromosome 2 (1 case).
Saliva samples were also collected from 49 healthy 3-15 year-old children and adolescents and from 11 of 20-42 year-old adults who were used as controls. The Iysoplate method was utilied to determine the Iysozyme concentrations in saliva samples. This test is based on the agar plate diffusion method first described by Osserman & Lawlor (1966)7. To prepare the agar plate, a 10 mg amount of Micrococcus Iysodeikticus (Sigma, M 3770, USA) was suspended in 10 ml of phosphate buffer (PB), and was added to a 10 ml solution of 2 % agarose, also diluted in PB, pH 6.2. Wells were made in the agarose to place the standard curve and saliva samples. For the construction of the standard curve, egg Iysozyme (Sigma, L-6876,USA) was diluted to 1 mg/ml in PB pH 6.2 and the fresh saliva samples were centrifuged at 23.15 x g for 10 min, the pellet was discarded and the supernatant was utilized. To quantify the Iysozyme content of saliva, 5 μg/ml of each saliva sample was transferred to each well. The plates were incubated 18 h at 24 °C and cleared zone ring diameters of Iysis were measured. A standard curve was constructed by relating the egg Iysozyme concentration and the cleared zone ring diameter of Iysis. The values were plotted on the graph to determine the Iysozyme concentration of the saliva samples. Graphpad Prism program was utilized to calculate these results.
Statistical analysis was carried out using variance analysis with three factors. It was performed with 95 % confidence limits and a probability value p < 0,05 would be considered as significant.
RESULTS
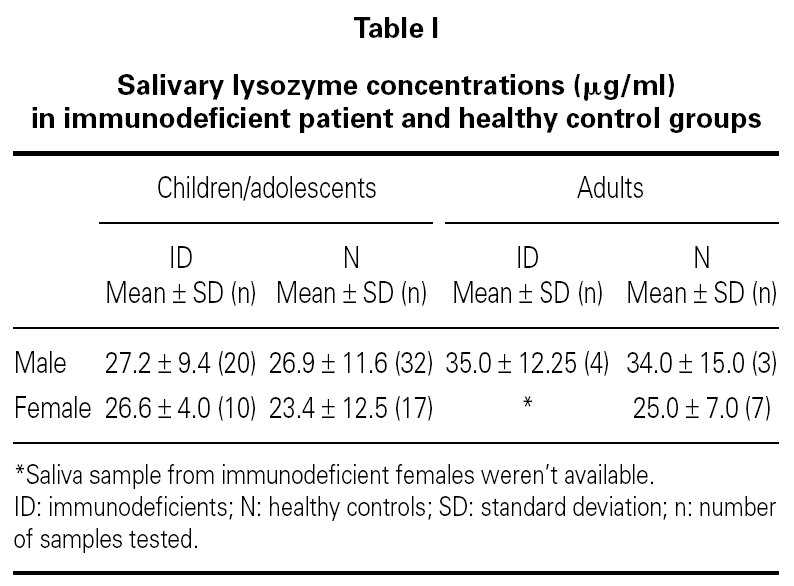
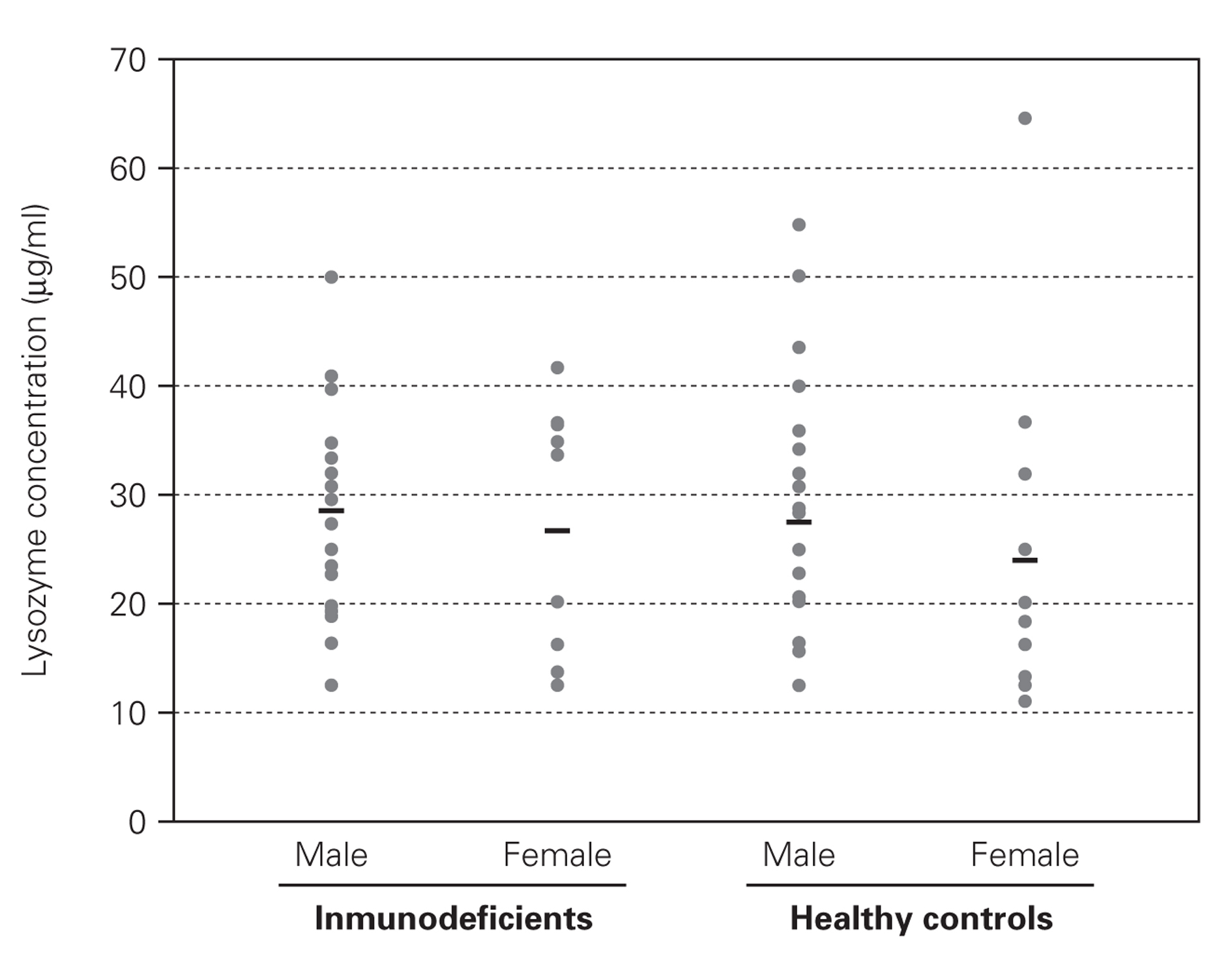
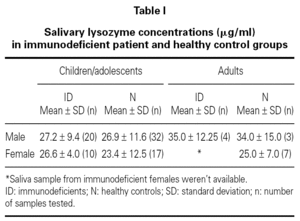
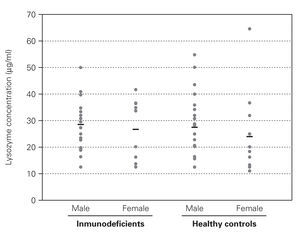
No difference was found between the Iysozyme levels in saliva of the immunodeficient patients and Iysozyme concentrations in the healthy controls (table I), regardless of age, sex and types of primary immunodeficiency (fig. 1). Variance analysis revealed that comparison among the groups failed to reach significance (age, p = 0.136; sex, p = 0.205; group, p = 0.663; age versus sex, p = 0.508; age versus group, p = 0.941 and sex versus group, p = 0.592).
Figure 1.--Salivary Iysozyme concentrations in male and female immunodeficient patients and in age-matched male and female healthy controls indicates mean values.
DISCUSSION
Innate immunity is increasingly recognized as crucial for the resistance to infection. Lysozyme could represent a contributory factor to mucosal immunity and to protection against Gram-positive bacterium in patients with severely impaired immune response8.
In this paper, it is demonstrated that salivary Iysozyme levels in patients with several primary immunodeficiencies are equivalent to those of healthy age-matched controls, including one patient with Chédiak-Higashi Syndrome, who was previously described as having low activity of this enzyme inside the macrophage9. Similar results have been reported by Kirstila et al (1994), who demonstrated that in 15 patients with Common Variable Immunodeficiency (CVI), Iysozyme and other innate immunity substances such as lactoferrin, salivary peroxidase, myeloperoxidase, hypothiocyanite, thiocyanate and agglutinins are equivalent to those found in healthy controls. Those authors suggest that some of these agents do, in fact, seem to be produced in higher amounts to compensate the low antibody titers. It is possible that the unspecific factors form a "backup system" in patients with CVI, in accordance with findings in human infants who are still physiologically immature with respect to immunoglobulins but not as regards to Iysozyme, total peroxidase activity or hypothiocyanite10. It was also described that concentration of salivary Iysozyme in HIV patients with oropharyngeal candidiasis was higher than the HIV ones without oropharyngeal candidiasis or the healthy control group11.
The results of the present work clearly show that salivary Iysozyme levels in primary immunodeficient patients are equivalent to those found in healthy controls, suggesting that this enzyme still represents a remaining (but not compensatory) mechanism, contributing to the protection of these patients against infections.
ACKNOWLEDGEMENTS
The authors are thankful to Dra. Eugenia Grillo Carnide from the Department of Pediatrics, Instituto de Criança. Hospital das Clínicas. São Paulo, Brazil. This work was supported by FAPESP (grants 02/13706-4).