The management of bronchiolitis is still controversial and to the best of our knowledge, no clinical trial with oral corticosteroids in outpatients has been carried out in developing countries. The objective was to compare the efficacy of a single dose of oral dexamethasone in infants with moderate to severe bronchiolitis presenting to an emergency department.
Material and MethodsA randomised, double-blind, placebo-controlled trial was conducted in Paraguay. At baseline, respiratory distress assessment instrument (RDAI), heart and respiratory rates, and transcutaneous haemoglobin oxygen saturation (SpO2) were recorded. Children received either a single dose of dexamethasone (0.5mg/kg) or placebo; and then both groups received two nebulisations with adrenaline. Respiratory status was recorded again after the 1st and 4th hours. The primary outcome was RDAI improvement at the 4th hour; and the secondary was the hospital admission rate.
ResultsDuring 5 months, 80 (33.3 %) out of 240 infants who consulted with acute respiratory illness fulfilled the inclusion criteria. During the trial 15 were excluded, therefore, 65 infants (33 in the dexamethasone vs. 32 in the placebo group) finished the study. Baseline characteristics and respiratory status were similar between groups. There were no differences in RDAI, heart and respiratory rate and SpO2 between groups after the 1st and 4th hours. The hospitalisation rate was similar between groups (21 % vs. 25 %, p=0.9, respectively), independently of the virus identified.
ConclusionsInfants with moderate-severe bronchiolitis who were treated with a single dose of dexamethasone did not significantly alter the rate of hospitalisation or respiratory status.
Bronchiolitis is a very common disease and the leading cause of hospitalisation for infants in developed and developing countries. In the US, almost 10 to 20 % of the infants with bronchiolitis required hospitalisation, and between 1980 and 1996 this rate more than doubled1,2. In Paraguay, bronchiolitis is responsible for 60 % of hospital admissions (unpublished data by the authors). Despite its burden in hospitalisation and health system costs; its treatment is still controversial3.
A recent meta-analysis comparing beta-2 agonists vs. placebo concluded that bronchodilators produce small short-term improvements in clinical scores but no improvement in oxygenation on outpatient and inpatient infants; and no reduction in the rate of hospital admission among outpatients4. Meta-analysis of epinephrine/adrenaline trials demonstrated the advantage of using epinephrine vs. placebo and vs. salbutamol in clinical score, respiratory rate and oxygen saturation in outpatients; however it did not decrease the hospital admission rate5. It is known that more than 25 % of infants hospitalised received therapy with corticosteroids, even though there is a lack of strong evidence to support their use. Garrison et al 6 in a meta-analysis of six trials of systemic corticosteroids on inpatients with bronchiolitis, showed a small benefit in clinical symptoms and length of stay, but the effect of corticosteroids disappeared when only studies of first-time wheezing were analysed.
Trials of systemic corticosteroids in outpatients showed contradictory results. Schuh et al 7 found that a single oral dose of dexamethasone improves symptoms and reduces the admission rate in infants with moderate-severe bronchiolitis compared with placebo. On the other hand, Corneli et al8 in a recent large trial failed to demonstrate any benefit of oral dexamethasone when used in this type of patients.
Furthermore, it has been speculated that in developing countries the treatment and outcome for bronchiolitis (due to the higher pneumonia and wheezing associated) should be different than in developed countries3. To the best of our knowledge, no clinical trial with oral corticosteroids in outpatients with bronchiolitis has been carried out in developing countries. The objective of the present study was to compare the efficacy of a single dose of oral dexamethasone in infants with acute moderate-severe bronchiolitis treated in an emergency department (ED) in a developing country. Our hypothesis was that children who received a single dose of oral dexamethasone results in clinical improvement and reduces hospital admissions.
Material and MethodsThis study was conducted in the ED of the Hospital General Pediátrico “Niños de Acosta Ñu”, Asunción, Paraguay, an inner-city facility which attends a socioeconomically disadvantaged population. Children 2–24months of age who came to the ED with their first episode of “bronchiolitis” (defined as respiratory distress with respiratory rate between 40-80/ min and wheezing; and within 7days after onset of a cold), were eligible for the study8. Exclusion criteria included clinical or radiological pneumonia; cardiopulmonary congenital malformations; bronchopulmonary dysplasia; cystic fibrosis; foreign body aspiration; neurological alteration; previous wheezing/asthma episode; inhaled or systemic corticosteroids used in the previous 15days; beta-2 agonists used within the previous 4 hours; antecedents of atopy (dermatitis or allergic rhinitis) in the child or parental asthma; and severe wheezing attack (respiratory rate ≥ 100/min and/or heart rate ≥ 200/min and/or shock or lethargy).
During five months, 240 infants with acute respiratory illness were attended in the ED; however, only 80 (33.3 %) infants (all of them mestizo) satisfied the inclusion criteria and were enrolled in the study. In all of the 80 children a chest X-ray was performed and it was seen by an independent radiologist to exclude the presence of pneumonia. X-ray criteria considered compatible with the diagnosis of pneumonia were the presence of alveolar infiltrates or lobar consolidation. At the enrolment, two study physicians (who were previously trained in study procedures and respiratory scoring and had an interclass correlation coefficient kappa of 0.65) determined the Respiratory Distress Assessment Instrument (RDAI) 9 —which is a scale of 0 to 17 and where higher scores indicated more severe disease—; respiratory and heart rates; and transcutaneous haemoglobin oxygen saturation (SpO2) measured by a pulse oximeter (Datex Tuffsat, Ohmeda, CO) at room air. Demographic data were also collected.
Patients were randomised to a double-blind, placebo-control study using a table of random numbers. The research pharmacy prepared the active drug (dexamethasone, Lab. Formula Magistral, Paraguay) and the placebo in identical sweet syrups and their bottles were labelled only with the randomised numbers. The study group received a single dose of 0.5mg/kg (1ml/kg) of dexamethasone whilst the placebo group received a single dose of syrup placebo (1ml/kg). Any episode of vomiting within 20min after administration of the oral study medication was recorded, but the dose was not repeated. Immediately after the dose, children from both groups received two nebulisations with 4ml of physiological solution and 1ml of L-adrenaline solution (1:1000, 1ml = 1mg) separated by 30min. Aerosol was generated by jet nebuliser (Micro-nebulizer, Brazil) powered by continuous flow of oxygen (6L) for 7min and delivered via a tightfitting face mask.
After one and four hours of the study medication administration, the patients were re-evaluated by two study physicians and the RDAI score, SpO2, respiratory and heart rates were again recorded. At the end of the fourth hour, the physicians decided whether to discharge or admit the patient to the hospital. The child was admitted if he or she had SpO2 ≤ 90 % and/or respiratory rate above normal values for age. The primary outcome of the study was a change in RDAI score at the fourth hour and the secondary outcome was the decrease in the hospital admission rate.
During the trial, additional oxygen was administrated to the patient if he or she had SpO2 less than 90 %; and also aspiration for cleaning the nose was carried out and antipyretic medication was provided when necessary. Nasopharyngeal swabs for respiratory virus (respiratory syncytial virus [RSV], adenovirus, influenza A and B) detection by indirect immunoflourescence were also performed. In the whole period of the trial, the investigators were blinded of the treatment administered. No systematic follow-up after the fourth hour was done.
This study was approved by the Hospital Ethics Committee and a written signed and fully informed consent was obtained from parents.
Statistical AnalysisTo evaluate differences between groups, the chi-square and t-test were used for categorical and for continuous variables, respectively. Changes in the RDAI score of 2 points were reported to clearly differentiate patients who required admission10; therefore, for the detection of this difference of 2 points, the number required per group was 27, with a statistical power of 80 % (beta = 0.20) and alpha level of 0.05, two-sided test, and a standard deviation of 2.6.
ResultsDuring the study period, 240 infants with acute respiratory illness were attended in the ED. One hundred and sixty infants did not meet the inclusion criteria (most of them had common cold, recurrent wheezing episodes, severe respiratory distress, or received oral bronchodilators before consulting). Among the 80 (33.3 %) infants who met the criteria and were initially enrolled, 15 were excluded during the process: 5 children had radiological evidence of pneumonia, 2 children quit the protocol before the first hour of the protocol, 3 presented vomiting within 20min after administration of the medication (2 from the dexamethasone and 1 from the placebo group) and parents of 5 children declined to participate. Of the 65 remaining infants, 33 were in the dexamethasone and 32 in the placebo group.
There were no significant differences in demographic characteristics between groups, the proportion of males and mean age were similar (58 % vs. 47 %, p = 0.4; and 7.3 ± 4 vs. 5.9 ± 3months, p = 0.1; for dexamethasone and placebo, respectively). However, infants from the dexamethasone group had significant higher weight than the placebo (8.098 ± 2.530 vs. 6.543 ± 1.670kg, p = 0.005).
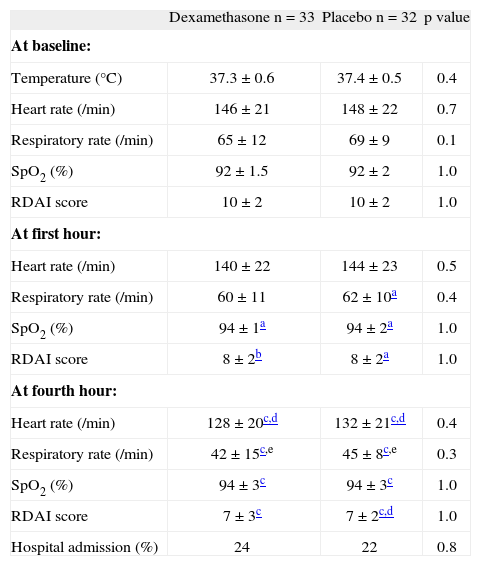
There were no differences in heart and respiratory rates, RDAI score and SpO2 at first and at fourth hour of treatment between the dexamethasone and placebo group (Table I). When we compared data among children inside each group or intra-group, the RDAI score decreased and the SpO2 increased significantly from baseline to the first hour of treatment in both groups; while the respiratory rate decreased more in the placebo group. These four parameters (heart and respiratory rates, SpO2 and RDAI score) changed significantly from the baseline vs. the fourth hour of treatment among children in both groups; and also heart and respiratory rates decreased significantly from the first to the fourth hour in both groups (Table I). At the end of the study (4th hour) 23 % of the infants required hospitalisation (24 % on the dexamethasone and 22 % on the placebo, p = 0.82, OR = 1.14, 95 %CI = 0.36-3.63). 42 patients would need to be treated (NNT) with dexamethasone to prevent just one hospitalisation.
Characteristics at baseline, first and fourth hours of observation between the two groups
| Dexamethasone n = 33 | Placebo n = 32 | p value | |
| At baseline: | |||
| Temperature (°C) | 37.3 ± 0.6 | 37.4 ± 0.5 | 0.4 |
| Heart rate (/min) | 146 ± 21 | 148 ± 22 | 0.7 |
| Respiratory rate (/min) | 65 ± 12 | 69 ± 9 | 0.1 |
| SpO2 (%) | 92 ± 1.5 | 92 ± 2 | 1.0 |
| RDAI score | 10 ± 2 | 10 ± 2 | 1.0 |
| At first hour: | |||
| Heart rate (/min) | 140 ± 22 | 144 ± 23 | 0.5 |
| Respiratory rate (/min) | 60 ± 11 | 62 ± 10a | 0.4 |
| SpO2 (%) | 94 ± 1a | 94 ± 2a | 1.0 |
| RDAI score | 8 ± 2b | 8 ± 2a | 1.0 |
| At fourth hour: | |||
| Heart rate (/min) | 128 ± 20c,d | 132 ± 21c,d | 0.4 |
| Respiratory rate (/min) | 42 ± 15c,e | 45 ± 8c,e | 0.3 |
| SpO2 (%) | 94 ± 3c | 94 ± 3c | 1.0 |
| RDAI score | 7 ± 3c | 7 ± 2c,d | 1.0 |
| Hospital admission (%) | 24 | 22 | 0.8 |
Numbers are expressed in % or mean ± SD.
SpO2: transcutaneous haemoglobin oxygen saturation; RDAI: Respiratory Distress Assessment Instrument.
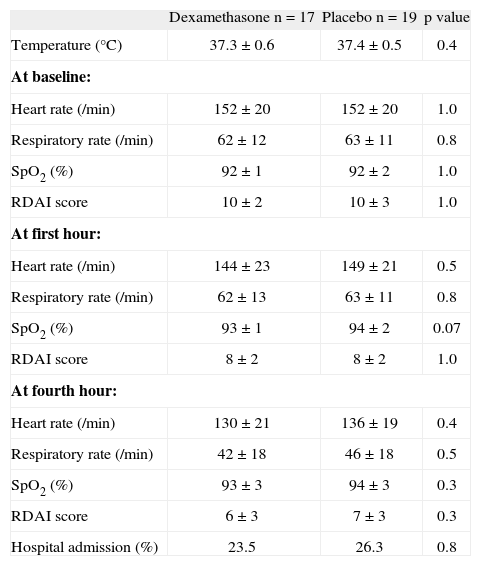
In 52/65 (80 %) infants the respiratory virus sampling could be done (29 and 23 infants from dexamethasone and placebo group, respectively). The number of virus identified between groups was: 17 vs. 19 for RSV; 3 vs. 0 for influenza A; 1 vs. 0 for influenza B; 3 vs. 1 for adenovirus, respectively. There were no viruses identified in 5 and 3 cases, respectively. There were marginally fewer positive RSV cases among infants from dexamethasone than from placebo group (58.6 % vs. 82.6 %, p = 0.063, respectively). However, when we analyse those 36 infants with positive RSV, there were no significant differences in RDAI score, respiratory and heart rate at baseline, at the first and fourth hour of observation between groups of therapy; although at the first hour the SpO2 in the placebo was marginally higher than in the dexamethasone group (Table II). The proportion of children with positive RSV who required hospitalisation was similar between the groups (23.5 % from dexamethasone vs. 26.3 % from placebo, p = 0.8).
Characteristics between the two groups at baseline, first and fourth hours of observation among children with positive RSV
| Dexamethasone n = 17 | Placebo n = 19 | p value | |
| Temperature (°C) | 37.3 ± 0.6 | 37.4 ± 0.5 | 0.4 |
| At baseline: | |||
| Heart rate (/min) | 152 ± 20 | 152 ± 20 | 1.0 |
| Respiratory rate (/min) | 62 ± 12 | 63 ± 11 | 0.8 |
| SpO2 (%) | 92 ± 1 | 92 ± 2 | 1.0 |
| RDAI score | 10 ± 2 | 10 ± 3 | 1.0 |
| At first hour: | |||
| Heart rate (/min) | 144 ± 23 | 149 ± 21 | 0.5 |
| Respiratory rate (/min) | 62 ± 13 | 63 ± 11 | 0.8 |
| SpO2 (%) | 93 ± 1 | 94 ± 2 | 0.07 |
| RDAI score | 8 ± 2 | 8 ± 2 | 1.0 |
| At fourth hour: | |||
| Heart rate (/min) | 130 ± 21 | 136 ± 19 | 0.4 |
| Respiratory rate (/min) | 42 ± 18 | 46 ± 18 | 0.5 |
| SpO2 (%) | 93 ± 3 | 94 ± 3 | 0.3 |
| RDAI score | 6 ± 3 | 7 ± 3 | 0.3 |
| Hospital admission (%) | 23.5 | 26.3 | 0.8 |
Numbers are expressed in % or mean ± SD
RSV: respiratory syncytial virus; SpO2: transcutaneous haemoglobin oxygen saturation; RDAI: Respiratory Distress Assessment Instrument.
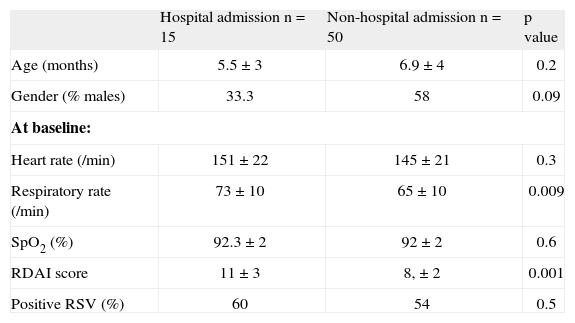
When we compare the children who at the end of the study (4th hour) required hospitalisation versus those who did not, those 15 children who were admitted (7 from dexamethasone and 8 from placebo group) had significantly higher RDAI score and respiratory rate at admission compared to those who did not require hospitalisation, regardless of the result of RSV (Table III).
Characteristics between infants who were admitted to the hospital at the fourth hour of observation versus non-admitted
| Hospital admission n = 15 | Non-hospital admission n = 50 | p value | |
| Age (months) | 5.5 ± 3 | 6.9 ± 4 | 0.2 |
| Gender (% males) | 33.3 | 58 | 0.09 |
| At baseline: | |||
| Heart rate (/min) | 151 ± 22 | 145 ± 21 | 0.3 |
| Respiratory rate (/min) | 73 ± 10 | 65 ± 10 | 0.009 |
| SpO2 (%) | 92.3 ± 2 | 92 ± 2 | 0.6 |
| RDAI score | 11 ± 3 | 8, ± 2 | 0.001 |
| Positive RSV (%) | 60 | 54 | 0.5 |
Numbers are expressed in % or mean ± SD.
RSV: respiratory syncytial virus; SpO2: transcutaneous haemoglobin oxygen saturation; RDAI: Respiratory Distress Assessment Instrument.
This randomised trial of 65 infants (less than 24months of age) with moderate-severe bronchiolitis who consulted to an ED in a developing country, showed that one single oral dose of dexamethasone (0.5mg/kg) did not significantly reduce the hospitalisation rate or improve the respiratory condition after 4 hours of observation compared to placebo. Those children who presented to the ED initially with higher respiratory distress (higher RDAI score and respiratory rate) were those who required hospitalisation, independently of the treatment received and the virus isolated.
These results agree with a recent large trial in the US on 200 infants (under 12months of age) with bronchiolitis where the rate of hospital admission between those who received a higher single dose of oral dexamethasone (1mg/ kg) was similar to the placebo (39.7 % vs. 41 %, p = 0.74, respectively); and also the respiratory status (including RDAI) after four hours of observation was also similar.8 On the other hand, a previous study on 70 Canadian infants (under 24months of age) with bronchiolitis showed a significant benefit of a single dose of oral dexamethasone (1mg/kg) in reducing the hospital admission rate vs. placebo (19 % vs. 44 %, respectively, p = 0.039), as well as improving their respiratory status7. In both studies the severity of the bronchiolitis was very similar to our study, e.g. the RDAI score at admission for the dexamethasone vs. placebo group were: 10 ± 2 vs. 10 ± 2 in our study, 9.0 ± 2.1 vs. 9.2 ± 2.4 in Corneli et al8 and 9.4 ± 2.3 vs. 10 ± 2.6 in Schuch et al7. Also in those three studies the response to prednisolone was measured at the fourth hour. The rationale for that period of observation is due to reasonable evidence of the benefit of corticosteroids after 2 to 4 hours of administration in asthma11–14 and also in croup15; and where the proposed biological mechanism includes upregulation of beta-2 receptors, mucosal vasoconstriction, and decrease in airway oedema.
However, a study from Turkey with 69 outpatients (2–21months of age) with mild to moderate bronchiolitis (mean RDAI at admission of 7.4) demonstrated that a single dose of intramuscular dexamethasone added to nebulised L-epinephrine or salbutamol therapy resulted in better outcome measures than bronchodilators alone only in the late phase (at the fifth day) but not in the first 24 hours16. Also, Csonka et al 17 in a clinical trial carried out in 123 Finnish children (6–35months old) with virally induced respiratory distress who consulted to the ED showed no immediately benefit of oral prednisolone (2mg/kg/d) on hospitalisation rate; and only 3-days of oral dexamethasone was effective to reduce the severity, the length of hospital stay and the duration of symptoms.
A meta-analysis on 13 clinical trials (three of them in outpatients) on corticosteroids in bronchiolitis showed no significant differences between corticosteroids and placebo in terms of respiratory rate; SpO2; length of stay; subsequent visits or readmission rates; and furthermore, there were no differences in the hospital admission rate among the outpatients18. In the AHRQ report19, the authors analysed five placebo-controlled studies of oral and two of parenteral corticosteroids (including dexamethasone) and only one study showed significant differences in the dexamethasone group. A previous meta-analysis done on six clinical trials of systemic corticosteroids demonstrated a small benefit of the corticosteroids, but this effect disappeared when outcomes were analysed separately or when only studies of patients with first-time wheezing were considered6. Therefore, the most recent recommendation of the American Academy of Pediatrics (AAP) 20 was that corticosteroids should not be used routinely for bronchiolitis, and they claim for the necessity to perform more studies. Indeed, our study shows that 42 patients would need to be treated (NNT) with single oral dexamethasone to prevent just one hospitalisation.
In the present study, as well as in the Shuch et al7 study, infants less than 24months of age with bronchiolitis were included, in contrast with the Corneli et al8 study in which only infants less than 12months of age participated. It has been speculated that bronchiolitis in children in their second year may be a mix of conditions, including early asthmatics with higher rates of atopy and greater association with asthma later. However, in that case we will expect that having older infants in our study may result in better response to dexamethasone than placebo yet this was not the case in our study; it is also important to remark that mean age was not significantly different in both groups.
Another difference between the present study and those done by Corneli et al8 and Shuch et al7 was that in our study both groups received two nebulisations of L-adrenaline solution (4mg), in comparison to four nebulisations of salbutamol (2.5mg) received7, and to any kind of bronchodilator8. However, none of the three studies was designed to examine the possible interaction between bronchodilators and dexamethasone. The AAP recommended a carefully monitored trial of alpha-adrenergic or beta-adrenergic and suggest continuing only if there is documented positive clinical response using objective evaluation tools20.
There were some limitations in the present study. First, we did not systematically follow the children after discharge from the ED because it was not part of the protocol (however, 25 infants from the dexamethasone and 18 from the placebo group were seen 24 hours after discharge by one of the investigators, and none of the patients required hospital admission). Second, some demographic/environmental characteristics (e.g. pets and tobacco exposure at home) were not registered and atopy markers were not done. Since our study included infants with moderate to severe bronchiolitis, the results cannot be generalised to more severe cases.
In conclusion, the use of a single-dose of oral dexamethasone (0.5mg/kg) did not significantly alter the rate of hospital admission or the respiratory status after four hours of observation in children younger than 24months of age presenting with a first episode of moderate-severe bronchiolitis.
Conflict of interestThe authors have no conflict of interest to declare.
We thank Mr. Anthony Carlson for his editorial assistance and to Lab. Formula Magistral, Asunción, Paraguay for providing the dexamethasone and placebo.