Skin testing remains an essential diagnostic tool in modern allergy practice.
A significant variability has been reported regarding technical procedures, interpretation of results and documentation. This review has the aim of consolidating methodological recommendations through a critical analysis on past and recent data. This will allow a better understanding on skin prick test (SPT) history; technique; (contra-) indications; interpretation of results; diagnostic pitfalls; adverse reactions; and variability factors.
Skin has an important physiological role in the internal balance homeostasis and constitutes a crucial barrier against external aggressions, with well-known immunological properties.1 It has been used by allergists for decades as an easily assessed laboratory of the immunological status of the individual.
The first skin testing technique was developed by Charles H. Blackley in 1865, a Manchester homeopathic physician with allergic rhinitis. He abraded a quarter-inch area of his skin with a lancet and then applied grass pollen grains.2 The so-called scratch test was later adopted by Schloss for the diagnosis of food allergy in children.3 Epicutaneous tests can be divided into scratch tests and prick/puncture tests. The first method, proposed by Blackley2, implied a linear scratch without drawing blood and could either be performed first, with the extract then dropped on the abraded skin, or be made through a drop of extract.4 Although it was used extensively in the past, this technique became progressively obsolete due to patient discomfort, poor reproducibility, possible residual lesions and newer and innocuous procedures.4 Therefore, scratch test is mentioned here for historical purposes only. It was Sir Thomas Lewis who, in 1924, first applied skin prick tests (SPT).5 Nevertheless, their generalised use in clinical practice only became a reality about 30years ago, as a result of technique modifications proposed by Pepys.6 For the purpose of this review and for easier comprehension, skin testing will be referred interchangeably as SPT, whatever device is used for its application.
In 1966, Ishizaka's work on immunoglobulin E (IgE) and immediate hypersensitivity reactions7 established the scientific corpus to what was done till then on a strictly empiric basis.
As written by Dr Walzer in 1974, “the fact that skin testing has not turned out to be a simple and completely reliable technique does not detract from the fact that, when it is intelligently and skilfully performed, it remains the most effective diagnostic procedure in reaginic allergic disorders”.8
The reliability of skin testing and proper documentation of test results are essential in allergy practice. A recent survey to all physician members and fellows of the American College of Allergy, Asthma and Immunology practicing in the United States detected a significant degree of variability regarding skin test devices, extract concentrations, interpretation and documentation of results and quality assurance procedures.9
This heterogeneity observed in clinical practice justifies the interest and relevance of the present review work. It is our aim to consolídate important technical recommendations, providing a new insight on the subject.
Skin prick testsGeneral considerations and indicationsIt is imperative that the clinician be fully aware of the clinical indications, correct technique, and interpretation criteria, as well as the risks and limitations of SPT. Skin testing should always be an adjunct to history and physical examination and not a substitute for medical evaluation.
SPT confirm the diagnosis of immediate hypersensitivity reactions.4,10
On skin level, the IgE-mediated immune response is dependent on both chemical and neurogenic mediators.11,12 After intracutaneous injection, allergens cross-link preformed IgE bound to the high-affinity receptor FcERI mast cells and a complex signal transduction cascade is activated. This eventually culminates in mast-cell degranulation beginning in seconds, with release of a variety of preformed inflammatory mediators. Among these are histamine – a short-lived vasoactive amine that causes an immediate increase in local blood flow and vessel permeability – and enzymes such as mast-cell chymase, tryptase and serine esterases.11 A wheal and flare reaction develops within minutes after superficial injection of antigen into the epidermis and lasts for up to 30 minutes. On activation, mast cells also synthesize and release chemokines, lipid mediators such as prostaglandins, leukotrienes and platelet-activating-factor, and additional cytokines such as interleukins 4 and 13 which perpetuate the Th2 response.11 These changes can sometimes be followed by a late-phase reaction (LPR), which is extremely rare and almost exclusive to patients sensitised to moulds, grass and parietaria pollens.13
In a positive reaction, histamine can be detected only at the centre of the wheal, not in the periphery. It is suggested therefore that after allergen challenge, the mediators released by the challenged mast cell induce an axon reflex by direct stimulation of c-fibres. This induces the release of neurogenic peptides and mast cell mediators from “the next” mast cell, becoming the major players in the immediate wheal and flare reaction.12
Skin testing can be used to select eviction measures and/ or specific immunotherapy.14
To optimally define test performance, a method should be reproducible and validated by comparison with gold standard methods. Direct challenge tests under supervision of a physician are appropriate ways to confirm or refute the validity of SPT. It provides objective evidence for sensitivity, specificity, predictive values and likelihood ratios. When compared to gold standard procedures, i.e. organ challenges such as nasal bronchoprovocation challenge or oral provocation challenge, SPT have demonstrated good results.15–18 The simplicity, rapidity of performance, low cost and high sensitivity make skin testing preferable to in vitro testing for determining the presence of specific IgE antibodies (sIgE). It is important to note the higher sensitivity of SPT when compared to sIgE dosing. Nevertheless, every positive result must be correlated with history and physical findings since a positive skin reaction does not necessarily imply the diagnosis of allergy.19
Interpretation of skin tests is highly dependent on the constitutive allergenicity, potency and stability of the allergen extract. For this reason, SPT sensitivity tends to be higher among aeroallergens, in particular pollens, house dust mite, fungi and certain epidermals.10
In clinical practice, skin testing has been extensively used for assessing sensitisation to inhalant allergens. SPT is useful to confirm or exclude a suspected diagnosis of allergic rhinitis, allergic conjunctivitis or asthma triggered by allergens 10,20,21 and to demonstrate sensitisation to inhalant occupational allergens.22,23
Previous observations suggest that skin test positivity at an early age is associated with subsequent development of rhinitis and wheeze.24–26 The role of allergic sensitisation as a cause of eczema is less clear.27
Skin testing in food allergy is common practice as well, although less reliable for commercial extracts of fruits and vegetables, as explained below.28 The clinical utility of SPT in patients with food allergy suspicion, especially infants and children, has been evaluated in various studies using oral food challenges and sIgE.
Most previous studies on food allergy obtained a concordance rate between SPT with commercial extracts and oral challenges from 60 % to 85 %28–32, specificity being generally lower, in the range of 40 % to 80 %.28 A negative result is useful to exclude type I reactions to food allergens (negative predictive accuracy > 95 %)32 but a positive result may or may not be associated with true clinical reactions. The overall concordance between a positive SPT and positive oral challenge differs between authors, but consensus exists regarding clear superiority of fresh food when compared to commercial extracts, as shown by Ortolani et al.33, Rosen et al.30, and Norgaard et al.34 With fresh food, sensitivity usually exceeds 90 % and can even reach 100 %.34 This is particularly important when a strong suspicion of food allergy subsists after negative results with commercial extracts. Fresh food testing makes use of a different procedure, the prick-prick technique.
Under carefully defined circumstances, SPT can also be used as a primary approach to drug and hymenoptera venom. In such cases however, intradermal tests are usually required for a correct diagnosis. For most chemicals associated with occupational allergy it is not indicated, with the exception of agents known to be implicated in IgE reactions, such as platinum salts, acid anhydrides, polyisocyanates, sulphonechloramide and succinylcholine analogues.35–37
TechniqueThe goal for the allergist is to perform skin testing with devices which minimise both false positive and false negative results while reducing patient discomfort. SPT should be a non-traumatic procedure (blood-free) and several sharp instruments such as a hypodermic needle, solid bore needle, lancet with or without bifurcated tip, and multiple-head devices, may be used.38
Historically, in the method first introduced by Pepys, the needle or blood lancet tip was inserted at an angle of 60.º to 70.º to the skin surface, gently lifting the superficial epidermal layers to create a small break in the skin.6
In 1979 a new method – puncture test – was proposed by Østerballe & Weeke, using a lancet with 1mm tip and shoulders to prevent further penetration.39 Lancets should be pressed with equal strength at 90º to the skin surface through a drop of extract or control solutions.4 This technique appears to be more precise than the original SPT method proposed by Pepys.40,41
Multiheaded devices are designed to first be dipped into the extract bottles, then applied to the skin in one step. They appear to be more painful than single devices but it is noteworthy that with a minimal increase in pain, as many as eight times more tests are applied, rendering multiheaded devices particularly useful in paediatric ages.38
Lancets should be sterilised, a fresh lancet for each prick, with normalised measures and each lancet should be used only once for each extract, in order to avoid unintentional pricks, blood borne infections and allergen contamination. Metal lancets with 1mm penetration limit are considered equally efficient and less painful than other synthetic devices with 1.4 or 1.6mm penetration limits.39 The penetration limit is therefore a determinant factor when considering test efficacy and patient comfort, rendering metal lancets preferable when compared to other synthetic devices.13 Nevertheless, an objective comparison has not shown a clear-cut advantage for any single or multitest device and optimal results can be obtained by choosing a single prick/ puncture device, and properly training its use.38,40,42
Antiseptic solutions are recommended before SPT and skin should be totally dry before procedure.43
Recommendations have been made regarding the appropriate placement of allergen extracts. The recommended distance for skin prick testing has varied between 2 and 5 cm44 and test sites should be marked with an appropriate code.4 It is possible, however, for a positive reaction to enhance false-positive skin reactions at an adjacent site, even over the range of 5cm.43–45
SPT are usually performed on the volar surface of the forearm, at least 5cm above the wrist and 3cm below antecubital fossae,4 the least and most reactive areas of the upper limb, respectively. The tests can also be done on the upper arm or the back, with special attention to avoid reactivity differences between locations.43 It should be taken into account that not only is the back 20 % more reactive than the forearm but specific locations on the back vary in reactivity as well.46.47 Therefore, a minimum of 2cm distance between each SPT should be adopted.
For an accurate interpretation of wheal and flare reactions to allergens, both positive and negative tests should be used. A negative control solution is required to evaluate unspecific reactions related to prick testing trauma (dermographism).4,48,49 A saline solution, phenol at 0.5 % or glycerine at 50 % are recommended.10
For positive control, histamine dihydrochloride 10mg/ml (54.3mmol/1), equivalent to 6.14mg/ml of histamine base, or codeine phosphate at 9 % can be recommended.49 Some authors advocate the use of histamine at 1mg/ml50; however, in a study by Morais de Almeida et al., the concentration of 1mg/ml consistently presented negative results in more than 10 % of the patients.13 Therefore, histamine at 1mg/ml should be definitely abandoned.
The prick-prick test requires a different procedure, pricking the food first, and then the skin, using the same needle; or pricking the skin through food in a single manoeuvre.51 Foods with a hard consistency, such as peanut, can be ground, diluted in buffered saline at 1/3 weight/volume (w/v), or 500mg of food to 1.5ml of saline.52
Dreborg recommends at least two parallel tests performed with the same material in every patient with the exception of infants, in order to assure precision, as single negative tests (5 %) will be obtained in sensitised patients even with skilled technicians.4 In duplicate tests, the diameter should not vary more than 1mm.53–56 Several publications have provided innovative methods to assure skin test validity. A suggested protocol for quality assurance testing and proficiency testing for SPT can be found in literature.10 In Europe, a coefficient variation of less than 20 % after histamine control test has been suggested57, whereas a recent Childhood Asthma Management Study considered a variation inferior to 30 %.58
Reading and interpretationThe size of the papule is of paramount importance in SPT. However, both erythema and wheal should be measured for proper interpretation.10
Østerballe and Weeke39 demonstrated that the wheal size with histamine peaks earlier (9–12min) than with allergens (13–16min). In a recent study, using laser Doppler flow imaging and scanning of drawn wheal sizes, the maximum histamine wheal size was reached at 20 minutes.59 We therefore propose a consensus reading time for both positive control and allergen reactions at 20 minutes post-prick.
A valuable option concerning appropriate documentation of skin test results consists in outlining the wheal and flare reaction with a felt-tip pen and transferring results with transparent tape to a blank sheet of paper.4,13
Various indices have been used for interpretation of skin reactions. The papule's area is the most accurate49,59 and can be evaluated by planimetry, either directly with image-processing programs or from a traced copy.13,60 The interpretation of the skin prick test is subject to inter-observer variation. To overcome this issue, computerised procedures have been proposed, allowing a more precise area evaluation.60,61 Other methods such as laser Doppler technique62 and ultrasound63 have been tested with success.
The size of the reaction can also be assessed using:
- •
minimal diameter;
- •
mean wheal diameter, calculated as the sum of the largest diameter and its largest orthogonal diameter divided by 2; or
- •
skin index, defined as the ratio of allergen wheal diameter divided by the histamine wheal size.
The SPT result should be considered positive if:
- •
minimal wheal diameter is greater than 3mm or;
- •
mean diameter is 3mm or larger; and/or
- •
skin index superior to 0.6.
Of the criteria explained above, mean wheal diameter is the most commonly used.
The skin reaction is considered positive if the wheal's area is 7 mm2 or higher, which corresponds approximately to a mean diameter of 3mm.9,40,57,64,65
The degree of erythema (flare) is considered to be a nonspecific reaction of the skin to the trauma of the puncture.66 Nevertheless, some authors consider a positive reaction if the mean flare diameter is over 10mm.49
The results obtained can only be correctly assessed and taken into account with valid positive and negative control reactions. Thus, histamine's papule mean diameter should be greater than 3mm and negative control should not exceed 3mm with erythema diameter inferior to 10mm. Devices that systematically produce negative control wheals over 3mm should be avoided.67 Stuckey et al. found that patients with more positive sensitisations and higher total IgE have larger histamine papules.68
Qualitative scoring (0 to 4+; 0 or +) is no longer recommended because of marked variability between observers.69,70
Wheal size has assumed greater diagnostic significance due to the positive correlation with clinical symptoms severity.71–74 Investigating graduated test responses and establishing probability decision points might improve diagnostic accuracy and predict positive reactions during organ challenge.75 In a previous study, especially regarding food allergy, Sporik74 defined specific wheal diameters as ‘100 % diagnostic’. In his work, cut-off values were proposed for cow's milk (≥ 8mm), hen's egg (≥ 7mm) and peanut (≥ 8mm), suggesting that children exceeding these limits are allergic to this specific food. These recent advances might obviate the need for oral challenge in the future.74–76 These cut-off points vary for different allergens, being more accurate for cow's milk and hen's egg than for soy or wheat. Additionally, different populations may exhibit significant variability. Even though there is a correlation between SPT result or sIgE and likelihood of a clinical reaction, sensitisation level does not always correlate with allergic manifestations.77–79
One study points to between 7.5 % and 19 % asymptomatic sensitisations among Finnish schoolchildren.80 Skin test reactivity to inhalant allergens is reduced in asymptomatic sensitisations when compared with symptomatic patients.81 Asymptomatic sensitisation is generally considered a premorbid state of allergic disease, and has been proven to be a risk factor for the development of allergic rhinitis in children and young adults.82,83 Bodtger et al., in a 3-years follow-up study, showed that adults with asymptomatic skin sensitisation to birch pollen have an increased risk (about 60 %) of developing hay fever.82
Several studies have demonstrated that positive SPT in infancy, especially to hen's egg, predicts subsequent presence of eczema in childhood.83–86 Thus, sensitisation in asymptomatic children can precede and predict the development of eczema.87
LimitationsIn the past, the manufacture of skin test solutions imposed important technical limitations. The recent availability of standardised commercial extracts constitutes a major achievement in allergy testing. Allergen extracts are complex mixtures derived from natural source materials and as such are prone to natural variation, requiring proper standardisation to ensure consistency and reproducibility. Some physicians report non-negligible variability between extracts from different manufacturers, easily attested in our daily practice.88,89 Quality of allergen extracts is dependent on several parameters such as raw material quality, proper test extractions, adequate processing and removal of low molecular weight components by dialysis or filtration.90 Stability, potency and allergen concentration are also determining.
The most internationally recognised way to express allergen extract strength is micrograms of major allergen because this appears to correlate well with overall biological potency of the extract.91 In-house references should be characterized with respect to dry weight, allergen complexity, major allergen content and IgE binding capacity. Biological activity should ideally be assessed in vivo, with skin testing.90–92 However, the methods used differ from manufacturer to manufacturer, making products from different companies impossible to compare.93
Non-related allergen mixtures may account for loss of biological potency as a consequence of excessive dilution or enzymatic deterioration of the epitopes. Time and higher temperatures can also accelerate the decay process. To assure stability, allergens are usually preserved with 50 % glycerine and stored under cold (4ºC).94,95
Recombinant allergens offer future interesting perspectives as in vivo diagnostic tools. These genetically engineered molecules appear to be highly specific, safe and biologically active. Their sensitivity, however, appears to be lower when compared to natural allergen extracts.89,96
Which allergens to test is a common doubt in daily practice. A recent survey performed in the United States showed that most allergists do not rely on history when choosing which allergens to use to perform skin testing.9
When considering inhalant allergy, several criteria should be thought-out before choosing skin testing reagents, such as botanical and aerobiological surveys. Flowering season, types and levels of pollens and spores along the year and peak days of pollination should be considered. Annual pollen sampling data in various countries are now available on-line.97,98 Air composition and concurrent allergy symptoms during recurrent seasons constitute the best indicators in the selection of appropriate outdoor aeroallergens for skin testing.10
The influence of pollen load is more evident in sIgE changes than on SPT reactions or clinical symptoms.99
Regarding food allergy, SPT can be performed both with commercial allergen extracts and fresh foods. Fresh food is often used as it more accurately reflects the patient's life. In a French study it was demonstrated that fresh foods were more reliable in food allergy diagnosis than commercial extracts.100 Commercial extracts of fruits and vegetables (e.g., apples, oranges, bananas, potatoes, carrots, and celery), are likely to lose biological properties with time, reinforcing the role of prick-prick method with fresh food.100 This technique is also valuable when there are differences in the allergenicity of different cultivar strains (e.g., apples) or when no commercial extracts are available.101
Although prick-to-prick tests are widely used, it is important to notice that they are not standardised, often give false-positive results, and still bear the risk of systemic reactions, as discussed in later sections.
Instead of fresh food, freezing aliquots may facilitate skin prick testing in particular cases. Freezing cow's milk and hen's egg at –20ºC has been tested and it does not alter the allergenic properties of each component.102 However, these results cannot be transferred automatically to other foods without further testing.
Furthermore, commercial extracts may produce false-negative results since storage, cooking or digestive process may induce immunological alterations in relevant allergens, rendering a particular food more allergenic than achieved by commercial extracts.10
The presence of active cutaneous lesions, as commonly observed in patients with active atopic dermatitis, impair the proper SPT reading, and constitute a contra-indication to skin test procedures.4,48,103 Nevertheless, SPT can be performed in eczematous infants since no lesions exist on testing area. This can be useful as infants with eczema in the first 2years of life with concomitant allergic sensitisation have a greater risk of childhood asthma and allergic rhinitis than infants with non-atopic eczema.104
Patients with dermographism should be excluded as it is difficult to distinguish between a true or false positive result, invalidating any conclusions.4
In such circumstances involving extensive skin disease; or in patients under skin test suppressive therapy (for example, antihistamines) that cannot be discontinued; uncooperative patients; or when the history suggests an unusually high risk of anaphylaxis from skin testing, sIgE immunoassays may be preferable to skin testing.
False-positive reactions can be due to skin trauma, mostly in patients with dermographism4, as explained above, but also to contaminated allergen extracts (occurring during extract preparation or simply for not changing lancets during SPT)105 or cross-reactivity phenomena.106 Cross-reactivity depends on the type of allergens involved, in particular their structural and sequential similarity.106 Pan-allergens responsible for cross-reactivity in vegetables are pathogen-related proteins (PRP) and profillins.107 For invertebrates, tropomyosin is the most implicated protein. For vertebrates, several allergens are implicated: parvalbumin (fish), livetin and ovotranferrin (egg and birds) and casein (milk).108
Extensive cross-reactivity has been described among aeroallergen-sensitised patients.10,109,109 House dust mites, epidermals, but most of all, pollens have been widely studied. Therefore, testing with multiple locally prevalent pollens may be required to avoid significant omissions. Cross-allergenicity among major classes of airborne fungi has not been well delineated so far.10
With regard to food allergy, we shall briefly mention the most interesting and relevant syndromes as it can be useful to better understand skin test results. Patients with: 1) birch apple; 2) artemisia-celery-carrot-spices; 3) grass-peach; 4) plantago-melon; 5) latex-fruits; 6) dust mites-seafood; 7) bird-egg; 8) pig-cat; 9) shellfish; 10) peanut, soybean and other legumes; 11) tree nuts; 12) rosaceous fruits; and 13) cereal grains can be expected to show cross-reactivity with SPT.
Concerning false-negative results, special attention should be given to patient's age, concomitant drugs and diseases such as HIV infection or chronic renal insufficiency, which may inhibit skin reactivity.10 Even when all quality parameters are considered, patients with evident allergic symptoms can still have negative SPT. It is important to consider that non-IgE mechanisms, impossible to be assessed by SPT, can be implicated in patient's complaints. Powe et al.110 demonstrated that inflammation in non-allergic rhinitis may be a consequence of localised IgE-mediated reactions, not involving systemic Th2 responses or atopy. Therefore, local IgE production in non-allergic patients could explain the presence of symptoms in SPT negative patients (localised mucosal allergic disease in the absence of atopy – “entopy”).110
Adverse reactionsIn the last thirty years, the occurrence of systemic reactions with SPT for inhalant extracts has decreased dramatically.111 Recent surveys indicate an overall risk inferior to 0.02 % for anaphylactic reactions to SPT, whereas IDT are more likely to induce systemic reactions.111 Most of the systemic reactions incited by SPT were related to fresh food (prick-prick tests with kiwi, fish, fresh pine nut and milk)112–114, latex (SPT with natural rubber latex and commercial extracts)115,116 and drugs (penicillin, amoxicillin).111 In a 12-year survey of fatal reactions (1990–2001), one fatality was confirmed after SPT with multiple food allergens (90 food prick tests were applied at one time in a patient with moderately persistent asthma).117
A retrospective review of medical records concerning SPT with foods corroborates the low rate of generalised reactions, as previously stated, and points out that all reactions in infants (n = 6) occurred under 6months of age and only with fresh food specimens.118
Special attention should be given to young children and pregnant women. Skin test duplication should be avoided in children with suspected food allergy (fresh food or commercial extracts), especially when suffering from extensive eczema.111 As for pregnant women, although SPT is not contraindicated, it is prudent to postpone such procedures and/or propose sIgE assays instead.111
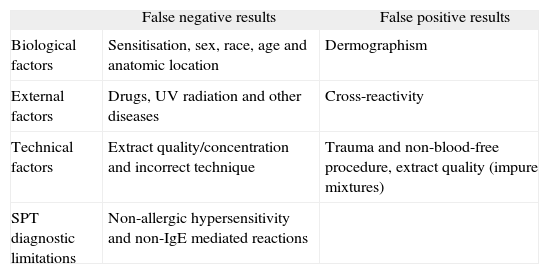
Variability factorsMultiple factors have been found to influence SPT results. These variability factors include technical issues, biological determinants and other external factors such as previous medication or infections (Table I).
False results in skin prick tests
| False negative results | False positive results | |
| Biological factors | Sensitisation, sex, race, age and anatomic location | Dermographism |
| External factors | Drugs, UV radiation and other diseases | Cross-reactivity |
| Technical factors | Extract quality/concentration and incorrect technique | Trauma and non-blood-free procedure, extract quality (impure mixtures) |
| SPT diagnostic limitations | Non-allergic hypersensitivity and non-IgE mediated reactions |
Skin reactivity is known to vary according to age: children, particularly under the age of 2years, are less reactive than adults.119 The prevalence of positive skin test results increases until the 2nd decade, with a slow decline above the age of 60years.120 In children with manifest allergy, however, skin has similar reactivity from 1year of age until puberty.4 Nevertheless, SPT tests can be used in infants as young as 1month, with a high degree of reliability, usually with more erythema than wheal reaction.119
Test results also depend on anatomic location since skin reactivity differs from region to region. In decreasing order, the degrees of reactivity are as follows: mid and upper back > lower back > upper arm > elbow > forearm (ulnar > radial) > wrist.47
Besides age and anatomic location, other biological and physiological factors may also influence skin test results, such as histologic qualities of the skin (vascularity, number of histamine receptors, mast cells, and dermal thickness).29 In one study, UV-B exposure was found to reduce skin reaction by as much as 48 %.121 Concerning racial factors, dark-skinned patients seem to have larger wheal-and-flare reactions, but some authors found Caucasians to be more reactive than non-Caucasians.10,46
Circadian rhythm has no influence on skin reactivity10,122 but some data show maximum wheal size during the night.46 Different studies identified a relevant increase in whealand-flare reaction in patients with allergic asthma and rhinitis after pollen season.123 On the contrary, the reduction in skin reactivity in sensitised subjects is a common finding after specific immunotherapy, either sublingual or subcutaneous.124,125
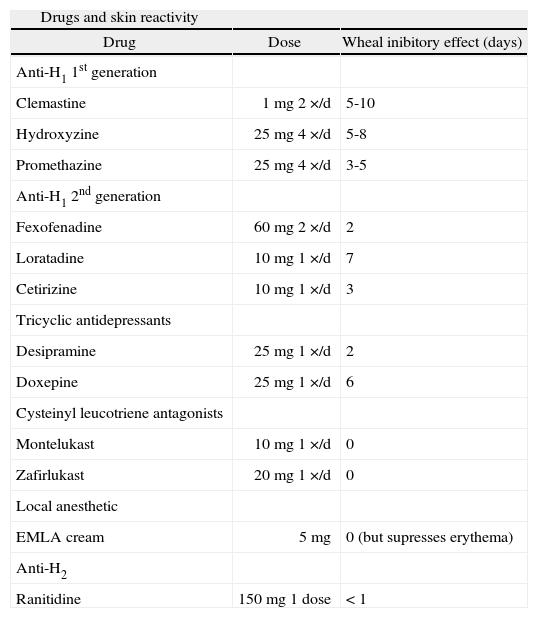
Concurrent drugs, in particular antihistamines (AH), tricyclic antidepressants and topical corticosteroids may affect the validity of skin testing.10 Different phamacodynamic models have been used to evaluate the degree and duration of drug suppression on skin reactivity, making direct comparisons unreliable (Table II).
List of drugs with skin inhibitory effect
| Drugs and skin reactivity | ||
| Drug | Dose | Wheal inibitory effect (days) |
| Anti-H1 1st generation | ||
| Clemastine | 1mg 2×/d | 5-10 |
| Hydroxyzine | 25mg 4×/d | 5-8 |
| Promethazine | 25mg 4×/d | 3-5 |
| Anti-H1 2nd generation | ||
| Fexofenadine | 60mg 2×/d | 2 |
| Loratadine | 10mg 1×/d | 7 |
| Cetirizine | 10mg 1×/d | 3 |
| Tricyclic antidepressants | ||
| Desipramine | 25mg 1×/d | 2 |
| Doxepine | 25mg 1×/d | 6 |
| Cysteinyl leucotriene antagonists | ||
| Montelukast | 10mg 1×/d | 0 |
| Zafirlukast | 20mg 1×/d | 0 |
| Local anesthetic | ||
| EMLA cream | 5mg | 0 (but supresses erythema) |
| Anti-H2 | ||
| Ranitidine | 150mg 1 dose | < 1 |
The histamine-induced wheal and flare model helps to identify the objective effectiveness of AH in humans, as well as their differences in the onset and duration of action. Several studies have employed this model to compare AH and assess their pharmacokinetic properties. When compared to other 2nd generation AH, such as desloratadine, levocetirizine appears to be more effective in inhibiting wheal and flare response.126,127 Ebastine, fexofenadine, cetirizine and mizolastine rank next and have similar skin effects. Superior efficacy of ebastine (20mg) was found in comparison to cetirizine (10mg) or loratadine (10mg) on the overall skin wheal response after single and multiple doses,127 with a longer-acting effect than fexofenadine as well.128
The general principle concerning first- and second-generation AH is to stop medication 2 to 3days before SPT, with the exception of cetirizine, hydroxyzine (5days)129, clemastine (5days)130, loratadine (7days)131 and perhaps others not yet studied. For this reason, and as it seems easier for the patient to remember, we suggest a one-week drug-free interval before skin testing.
Many patients who require SPT cannot deal with pruritus without taking AH. In a recent work by Danarti et al132 topical AH can be used in such circumstances. Because of their short duration of action (< 180 minutes), these drugs can be used in patients who need antihistamines but are scheduled to undergo skin prick testing after a few hours, without influencing the patient's skin response.132
Doxepin, a tricyclic antidepressant, and anti-H2 drugs can also cause false-negative results for as long as 6 days133 or 24h134, respectively.
Systemic corticosteroids do not inhibit skin reactivity when used for short term therapy (i.e. 30mg prednisone a day for 1week).135 When used for longer periods, conflicting results have been obtained, recommending a more critical analysis.10 Topical steroids should be discontinued 2 to 3weeks before testing as prolonged use (over 3weeks) can suppress wheal reaction in the application sites.136
Bronchodilators, epinephrine and theophylline do not significantly suppress skin reactivity.137 In the case of cysteinyl leukotrienes antagonists (e.g. montelukast and zafirlukast)138 or EMLA cream139, no significant effect on wheal-and-flare reaction has been described either. Concerning intranasal topical AH (e.g. azelastine) results are somehow contradictory and discontinuance is recommended for a 48h minimum period.10,140
Papule size depends as well on allergen concentration and number of allergens tested for which the patient is sensitised. Some authors have studied these variables and calculated, as an example, that the wheal diameter increases 1:5 times (area 2:5 times) if the allergen concentration increases 10 times.53,54 In polysensitised individuals, simultaneous prick testing with multiple allergens can induce additive histamine release from cutaneous mast cells. In vivo and in vitro studies suggest an additive effect of multiple proteins (allergens mixture) on histamine release from cutaneous mast cells, causing mean wheal diameters larger than obtained with single allergens.141
Future directionsSkin testing remains an essential diagnostic tool in modern allergy practice. Allergen extracts have experienced great progress in recent years but a long way remains ahead. Many allergens have yet to be characterized. The quality of extracts still needs further advances, with criterious allergen selection and biologic potency assessment. The capacity to differentiate between clinically irrelevant and relevant sensitisations constitutes an important motivation to future investigations. The definition and use of recombinant allergens promises to lead to an improvement in this area, eliminating diagnostic errors due to cross-reactivity phenomena.