INTRODUCTION
It is widely accepted that allergic disease is increasing, particularly in the case of type I allergies. Treatment by immunotherapy for type I allergies may be a particularly useful strategy, not only to provide long-term relief of symptoms of hayfever but also to curtail the development of more serious conditions such as bronchial asthma in younger patients1. Sublingual immunotherapy (SLIT) is a relatively new form of immunotherapeutic treatment which is increasingly prescribed for a number of reasons. Regarding safety, an analysis of sublingual/swallow SLIT in eight clinical trials found no serious side effects associated with treatment of a total of 690 patients2. Administration therefore need not be performed in a specialist clinic, and is frequently permitted at the patient's home. An EAACI consensus paper reviewing the treatment of allergic rhinitis found SLIT to be suitable for both adults and children3. A recent report from the Allergic Rhinitis and its Impact on Asthma (ARIA) workshop in collaboration with the World Health Organisation (WHO) recommended SLIT as an evidenced-based treatment for allergic rhinitis and conjunctivitis4. Compared with conventional subcutaneous immunotherapy, SLIT is more acceptable to many patients (particularly children) who may be inhibited by repeated needle injections. The employment of this treatment has however met with a guarded response by some clinicians because of doubts over the potential efficacy which, until recently, has not been widely reported; furthermore, the mechanistic action of sublingual immunotherapy is only partially understood. A local immune response is certainly suggested by rodent studies describing the activities of antigen presenting cells in the oral mucosa5,6. The number of documented clinical SLIT studies has also increased, now providing more convincing evidence of success. Recently, long-term efficacy of SLIT with house-dust mites was shown in asthmatic children7. A rigorous analysis of efficacy in 22 SLIT trials has confirmed that symptoms and medication requirements for patients may both be significantly reduced whilst retaining a high level of safety8. Thus SLIT may no longer be regarded as a relatively experimental clinical treatment, and is now becoming viewed as a serious and particularly safe alternative to injection immunotherapy.
As further evidence to improve acceptance of SLIT, we report the results of a post-marketing surveillance study of the efficacy and safety of SLIT administered within one year to a wide age range of patients treated with a comprehensive selection of allergen preparations appropriate for different inhalant allergies. The administration of the SLIT was via the "sublingual-swallow" method for all patients.
PATIENTS AND METHODS
Patients
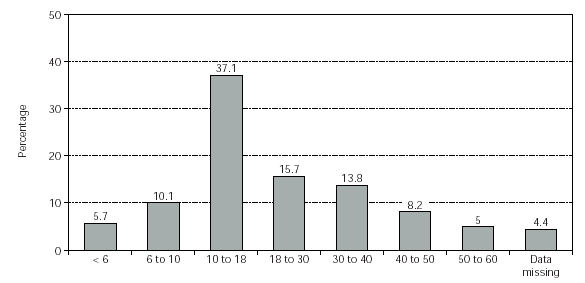
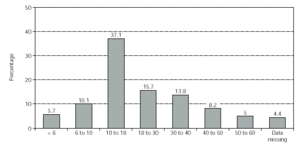
All patients in this multi-centre study were recruited from 42 centres in Germany (n = 159, 81 males and 78 females). The observation time of this study was from June 1999 to June 2000. The age distribution is shown in figure 1 (mean age = 21.7 years; median age = 16.5 years).
Figure 1.--Patient demographics: distribution of ages (years). No. of patients = 159.
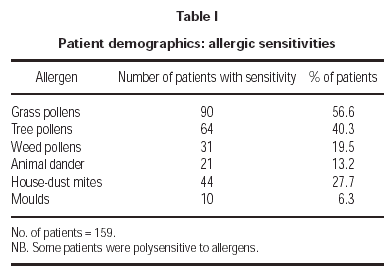
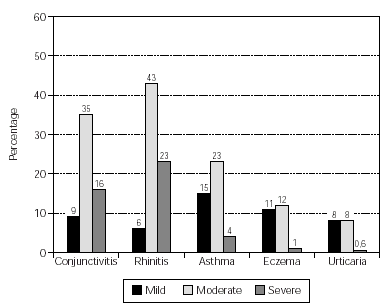
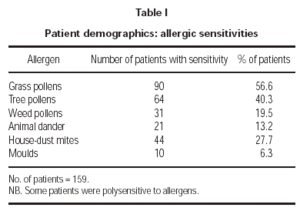
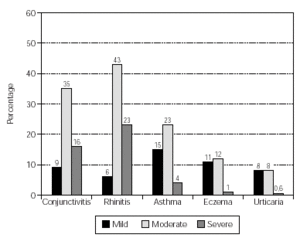
The allergic sensitisations of the patients are shown in table I. These were confirmed by clinical history and symptoms and a positive skin prick test (wheal diameter ≥ 3mm). Some patients were additionally diagnosed by RAST (class ≥ 2) and/or provocation tests. The patient symptoms included conjunctivitis, rhinitis, asthma, atopic eczema and urticaria. The occurrence and intensity of these symptoms are shown in figure 2.
Figure 2.--Patient demographics: allergic symptoms. No. of patients = 159.
Medication
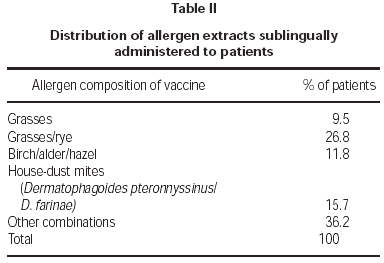
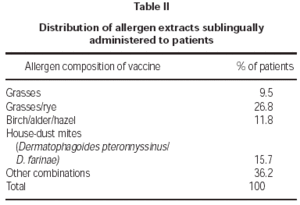
Patients were prescribed allergen vaccines corresponding to their sensitivities, as summarised on table II. Some combinations of allergens were used, but combined seasonal and non-seasonal allergens were not permitted (e.g. pollens with house-dust mites or animal dander) and not more than four different allergens in total.
All patients were treated with a standardised aqueous allergen extract in glycerol (ORALVAC®, Bencard Allergie GmbH, Munich, Germany) using three concentrations at 100 Standardised Oral Units (SOU)/ml, 1.000 SOU/ml and 10.000 SOU/ml. The dose was held under the tongue for approximately 1 minute before being swallowed. The investigator determined the dosage, the interval between medication, and the duration of treatment in accordance with the individual situation of the patient. Typically, initial course therapy was given in daily doses up to a period of four weeks. This would begin with one drop of the lowest concentration and end with sixteen drops of the highest concentration. Following this, a maintenance dose of 16 drops of the highest dose (10.000 SOU/ml) was administered 3 times a week for typically 4-6 months. Patients with pollen allergies were not treated during the respective pollen seasons.
Efficacy assessment
Efficacy was measured in two ways. Firstly, the consumption of anti-allergic medication was compared before and after therapy. The frequency of use was divided into categories of "regular", "often", "occasional" and "none". Secondly, a global assessment of efficacy was made by the physician, categorised as "very good", "good", "moderate", "unchanged" or "worsened".
Statistical significance was calculated using the Cochran-Mantel-Haenszel test.
Tolerability assessment
The frequency and intensity of local or systemic reactions was documented during treatment, together with a description of the symptoms by the treating physician.
Taste
The patient's opinion of the taste of the medication following initial therapy was recorded in categories of "very good", "good", "moderate" and "poor".
Acceptance
Physicians recorded the acceptance of therapy in categories of "very good", "good", "moderate" and "poor".
RESULTS
Efficacy
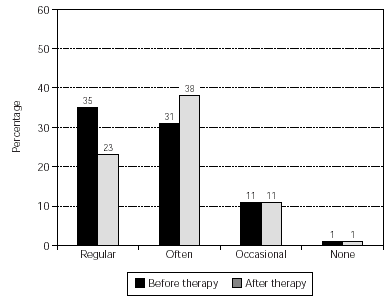
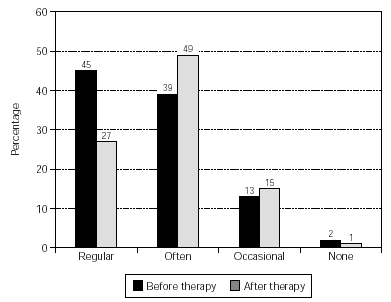
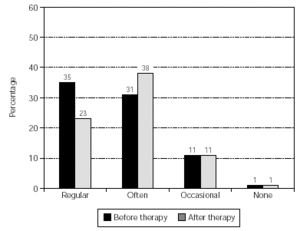
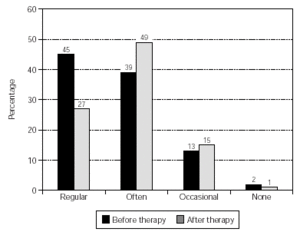
The overall use of medication was reduced significantly (p = 0.023) and the results are shown in figure 3. Also, the pollen-sensitive patients (grasses, rye, birch, elder, hazel, mugwort, plantain) were analysed as a sub-group, providing a further significant result (p = 0.016) (fig. 4).
Figure 3.--Consumption of anti-allergic medication by all patients. No. of patients = 159, p = 0.023. NB Data from some patients was not obtainable (before therapy, 34 patients; after therapy, 43 patients) and these cases were set as "not changed" for the calculation of significance.
Figure 4.--Consumption of anti-allergic medication by pollen-sensitive patients. No. of patients = 102, p = 0.016. NB data from some patients was not obtainable (before therapy, 1 patient; after therapy, 8 patients) and these cases were set at "not changed" for the calculation of significance.
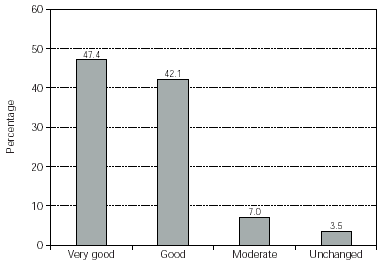
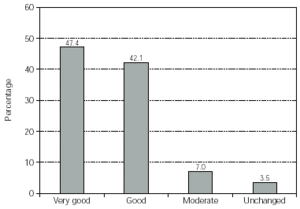
The therapeutic effect of the treatment was assessed by the physicians for the majority of the patients (some data was not recorded). The most frequent assessment was "very good". In total only 4 patients (3.5 %) found no benefit from the therapy (fig. 5). No case was documented as worsened after therapy.
Figure 5.--Physician's assessment of therapeutic effect. No. of patients = 114.
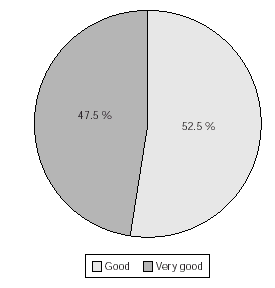
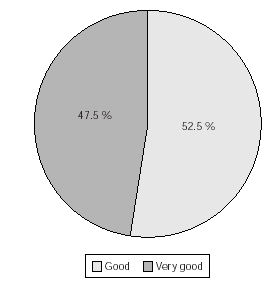
The taste of the medication (raspberry flavour) reported by patients was found as very good (47.5 %) or good (52.5 %) of the patients during maintenance therapy (fig. 6).
Figure 6.--Patients estimation of taste of sublingual vaccine during maintenance therapy. No. of patients = 120. N.B. 39 patients declined to comment, but no negative opinions recorded.
The acceptance of therapy was very high (very good, 67.2 %, good, 29.4 %). Moderate acceptance was reported for only 1 patient (0.8 %) and poor acceptance in 3 patients (2.5 %).
Tolerability
For 10 (6.3 %) of the 159 patients, a total of 12 adverse events (8 local, 4 systemic) were reported. No adverse event was regarded as serious or severe. Seven local reactions consisted of a prickling sensation or a swelling in the mouth. In one case itchy eyes were recorded as a local reaction. Of the 4 patients with systemic reactions, three presented with allergic rhinitis and one with an allergic exanthema, the latter symptom being possibly related to immunotherapy. This patient was given an antihistamine, the only occasion whereby medication was provided in the study. No premature termination of treatment was made as a result of side-effects.
DISCUSSION
Efficacy of an allergy vaccine may be measured in a number of ways, and it is believed that one of the most accurate parameters is consumption of anti-allergic medication following therapy. In this study, the efficacy of SLIT in the practice of allergologists was based on the frequency of use of symptomatic medication. Some studies have employed more sophisticated point-scoring systems giving variable scores for different drugs but it may be argued that this more detailed methodology can be arbitrary and misleading9.
The data presented here provided a statistically significant reduction in medication for all allergens (p = 0.023). A large number of patients were sensitive to grass, tree and weed pollens (table I) so there was a bias of pollen sensitivities. This probably reflects typical demographic characteristics of allergy patients and so it is not an unusual or unreasonable situation. The bias however may possibly be exaggerated because several patients were not evaluated for anti-allergic medication (fig. 3). In contrast, the large sub-group of patients sensitive to pollens was clearly defined with few non-evaluated patients and provided convincing evidence of therapeutic success (p = 0.016) (fig. 4). The latter results endorse the conclusions from a previous study employing the same SLIT vaccine for grass/rye and birch pollen allergic patients10.
The global assessments by the physician reinforced the reduced medication data, recording over 96 % of the evaluable patients with a beneficial outcome.
The median age of the patients was 16.5 years, indicating a trend towards younger patients, which is probably a reasonable reflection of typical age distribution in the practice of allergologists.
The importance of immunotherapy for younger children should not be underestimated, with the real potential of slowing down or even halting the "allergic march"1. Although it may seem trivial to some, the taste of the administered vaccine must be acceptable to gain patient compliance; this is an important issue not only for children. It was thus very encouraging that the taste was well-received, and in fact no patients complained of the taste being poor. This finding is supported by the high acceptance of therapy by the patients.
The tolerability of the treatment was high, with no serious or severe adverse events from any patient. The local reactions were generally prickling sensations or swelling in the mouth, which is a particularly common effect of SLIT. Of those few patients with systemic reactions, three developed allergic rhinitis and one an allergic exanthema. No wheezing or gastrointestinal symptoms were observed, although these have been reported in a number of other sublingual-swallow immunotherapy studies2. No premature termination of treatment due to side effects was made.
SLIT has shown good success in this study after a relatively short treatment period of up to 6 months; it is well-known that many clinicians advise a 3-5 year duration of immunotherapy courses11r. Thus with prolonged treatment even better results may be expected.
This study has demonstrated sublingual immunotherapy to be efficacious and safe when in routine use by allergologists. In particular, the favourable safety profile should contribute to establish SLIT as a valuable alternative to subcutaneous immunotherapy in the future.