To the Editor:
Many breast cancers have oestrogen receptors and their growth can be stimulated by oestrogen. In postmenopausal women, the principal source of circulating oestrogen is the conversion of adrenally-generated androstenedione to oestrone by aromatase in peripheral tissue, with further conversion to oestradiol. Treatment of breast cancer has included efforts to decrease oestrogen levels, by ovariectomy premenopausally and by use of anti-oestrogens and progestational agents both preand post-menopausally. These interventions lead to decreased tumour mass or delayed progression of tumour growth in some women1.
Anastrozole (AN) is a potent and selective third generation reversible nonsteroidal aromatase inhibitor2,3. It lowers serum oestradiol concentration and has no detectable effect on the formation of adrenal corticosteroids or aldosterone. It has been shown to reduce intratumoural oestrogen levels1. AN has good oral absorption, with maximum plasma levels reached within 2 hours of intake. It is extensively metabolised in liver (85 %) via N-dealkylation, hydroxylation and glucoronidation1. The most common adverse effects reported include: hot flushes (27 %); nausea (19 %); fatigue (16 %); joint pain and stiffness (12 %); bone, chest and back pain (11 %); cough (11 %), pharyngitis (10 %) and dyspnoea (10 %)1.
Third generation aromatase inhibitors are considered the most effective first-line endocrine treatment of advanced endocrine-responsive breast cancers since superior antitumoural activity has been repeatedly demonstrated versus tamoxifen in large phase III randomised trials in postmenopausal women4–7.
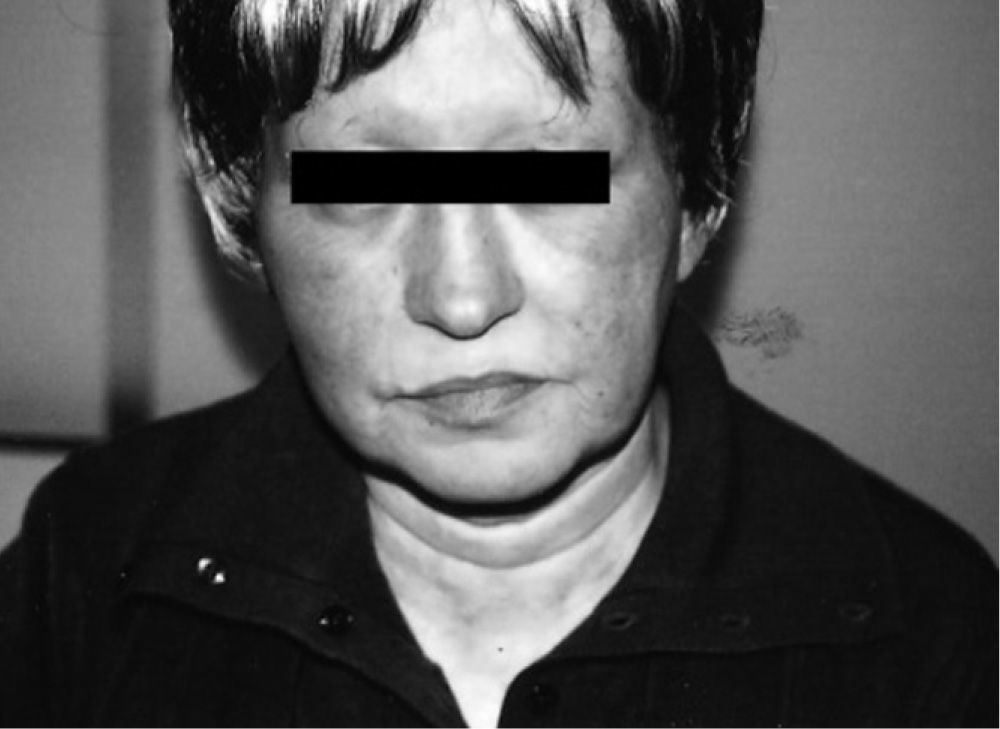
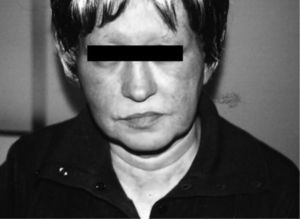
The authors report the clinical cases of two patients (55 and 59 years old) with advanced breast cancer, submitted to surgery, chemotherapy (adriamycin, cyclophosphamide and docetaxel) and radiotherapy, always with good tolerance. In November 2006 they began adjuvant treatment with AN 1mg/day. One of the patients referred a burning sensation on her face, macular exanthema, conjunctival hyperaemia, periorbital oedema, and watery discharge (fig. 1), three days after beginning the treatment. Due to progression of cutaneous symptoms to the neck and upper thorax, AN was discontinued on day 9 and oral antihistamines and corticosteroids were started with total resolution of symptoms.
The second patient referred macular exanthema on the face, neck and thorax two months after the treatment was started. AN was interrupted with total resolution of symptoms without any treatment.
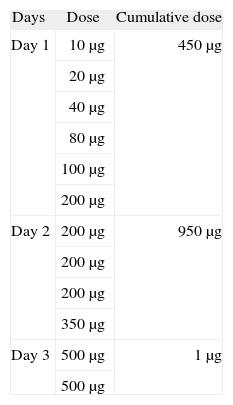
Patch tests with 10 % AN in white petrolatum were performed in both patients with negative results. Two exposed controls were also negative. Alternative treatment with tamoxifen was not possible because of its cardiotoxicity as one of the patients has heart disease. Letrazole, an alternative drug for both patients, was not available in our hospital. It was at this point that desensitisation to AN was proposed and performed. The desensitisation procedure (3-day protocol) was performed in the day care Hospital beginning with a 10μg dose, progressing until the cumulative dose of 1mg was achieved (Table). The patients continued taking AN 1μg/day without any reactions. They have been followed on monthly basis consultations without any symptoms.
All drugs can induce hypersensitivity reactions, which can limit the use of essential drugs in the treatment of serious diseases like cancer. The choice of an alternative chemotherapy regimen is often limited by tumour sensitivity, presence of comorbidities or the availability of alternative drugs.
Drug desensitisation can be defined as the induction of a state of unresponsiveness to a compound responsible for a hypersensitivity reaction. The induction of temporary unresponsiveness to drug antigens allows patients to be treated with medications to which they have presented hypersensitivity reactions8. This is achieved by gradual reintroduction of small doses of drug antigens at fixed time intervals, allowing the delivery of full therapeutic doses and protecting patients against previous severe drug reactions.
The desensitised state can only be maintained by continuous administration of the drug9. In spite of the amount of literature on drug desensitisation, the mechanisms behind this procedure are still not completely understood.
With regard to chemotherapy drugs there are some published data about successful desensitisation protocols to taxenes, platinum salts, L-asparaginase and monoclonal antibodies involving anaphylactic or anaphylactoid reactions8. To date no desensitisation protocols have been published on third generation aromatase inhibitors like AN.
In these case reports both patients referred late reactions to AN with mainly cutaneous symptoms. To clarify the underlying mechanism of these reactions, patch tests with 10 % AN in white petrolatum were performed with negative results. This could have several explanations: the final responsible agent for the reaction could be a metabolite of AN; poor penetration of the drug into the epidermis; low drug concentration, use of inappropriate vehicle10,11 and immunosuppression state of patients. Although no immune mechanism could be proven in our patients, a desensitisation procedure was tried with success. This result reinforces the fact that desensitisation to drugs that induce delayed cutaneous hypersensitivity reactions is possible and safe, as we have observed many times in patients with HIV infection and delayed cutaneous reactions to cotrimoxazol.