Although solid cohort studies confirmed a preventative role for the anti-oxidant vitamin D in allergic asthma, a limited number of studies focused on allergic rhinoconjunctivitis (ARC). Here, we aimed to determine 25-hydroxycholecalciferol levels in tear and serum in young allergic rhinoconjunctivitis patients as compared to their apparently healthy matched controls.
MethodsIn total, 22 children with allergic rhinoconjunctivitis and 31 healthy control subjects underwent serum total IgE and 25-hydroxycholecalciferol measurements. Tear levels of 25-hydroxycholecalciferol were also determined in both groups.
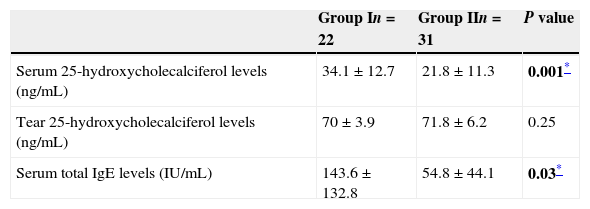
ResultsThe mean serum total IgE level in the ARC group (143.6±132.8IU/ml) was significantly higher than that in the control group (54.8±44.1IU/ml; p=0.03). Serum 25(OH)D levels were significantly higher in the ARC group (34.1±12.7ng/ml) than in the healthy controls (21.8±11.3ng/ml; p=0.001).
ConclusionsTo our knowledge, this is the first reported study to show an association between serum 25-hydroxycholecalciferol and ARC in a childhood group. Higher levels of serum 25-hydroxycholecalciferol in children with allergic rhinoconjunctivitis may indicate a possible aetiopathogenic mechanism in the development of allergic rhinoconjunctivitis. This is also the first report to examine tear fluid vitamin D levels in paediatric ARC patients.
Allergic rhinoconjunctivitis (ARC) is one of the most common chronic allergic conditions and is seen in all age groups.1 The prevalence of ARC has been increasing, affecting significant percentages of children group over recent decades.2 ARC is an inflammatory response of the conjunctiva to allergens, such as pollen, animal dander, and other environmental antigens. Redness, itching, and lacrimation are the most consistent symptoms. The aetiopathogenic mechanism of ARC involves a complex interplay of the conjunctiva and a dysregulated immune response.
Previous studies showed that vitamin D could be associated with allergic conditions.3,4 However, results regarding the relationship between vitamin D and allergic diseases including asthma, eczema, or rhinitis have been conflicting.5–7 Given the results of previous studies showing a possible role of vitamin D in allergic diseases, it seemed reasonable to expect that vitamin D status could be associated with ARC aetiopathogenesis, risk, or severity. However, to our knowledge, no reported study has examined the association between tear and serum 25-hydroxyvitamin D levels and ARC, especially in children. Thus, the aim of the present study was to investigate any association between plasma and tear fluid vitamin D status and allergic rhinoconjunctivitis in children.
Materials and methodsThe study was approved by the local ethics committee and followed the Declaration of Helsinki for research involving human subjects. Written informed consent was obtained from all participants or their parents.
Children with ARC and healthy control subjects of similar age and gender were included in the study. The diagnosis of allergic rhinoconjunctivitis was based on a typical clinical history and an ophthalmological examination. We asked about demographic, ophthalmological, and allergic history at the inclusion visit. Exclusion criteria were the presence of systemic diseases or ocular disease other than allergic conjunctivitis (except refractive error). Patients taking any topical and/or systemic anti-allergic drugs or vitamin D supplements were excluded.
The study was carried out in the western Black Sea Region of Turkey, Bolu, which lies in mountainous terrain with an altitude of 725m and is 30°32′east, 40°06′north. The percentage of coverage by forests is approximately 60% of the territory. According to the Turkish Directorate of Meteorology, the average temperature in the study period was 11.9°C daily. Participants were recruited consecutively from the paediatrics and ophthalmology departments in April and May, during the peak spring allergy season. An experienced paediatrician (SBG) evaluated all participants, applying the exclusion criteria and obtaining a detailed allergic history of the individuals and their parents, including asthma, allergic rhinitis, allergic rhinoconjunctivitis, and atopic dermatitis. After this paediatric evaluation, all study participants underwent dermatological and ophthalmological examinations.
Tear collectionWe decided to include children of seven years or older so as to obtain samples more readily. To eliminate diurnal variation in tear constituents, all samples were collected in the morning hours without previous topical anaesthesia, as described previously.8 All tear samples were collected in 20μl sterilised glass microcapillary tubes by capillary suction from the lateral canthus of each eye. All sampling procedures were standardised and were performed by one experienced investigator (ME) to minimise variability caused by the sampling method. Then, each sample was placed in a 1-ml microcentrifuge tube (Eppendorf, Fremont, CA, USA) and stored at −80°C until analysis.9
Serum collectionAfter tear sampling, blood from the forearm vein was collected into 5-ml Vacutainer tubes with no anticoagulant. The blood samples were centrifuged (1000×g, 15min, 4°C) to separate serum. Serum was removed and immediately stored at −80°C until analysed.
Biochemical measurementsTear and serum 25-hydroxycholecalciferol and total IgE measurements were performed using enzyme-linked immunosorbent assay (ELISA COBAS reagent kit; COBAS 6000 analyser series, Roche Diagnostics, Basel, Switzerland). Serum Vitamin D3 or total IgE Levels beyond the upper measurable level, diluted with saline then multiplied with dilution factor. 25-Hydroxycholecalciferol functional sensitivity is 4.01ng/ml. Its measuring range is 3.0–70ng/ml. Total IgE functional sensitivity is 0.100IU/ml. Its measuring range is 0.100–2500IU/ml.
Statistical analysisAll analyses were performed using the SPSS software (ver. 15.0 for Windows; SPSS, Inc., Chicago, IL, USA). Data are shown as means±standard deviations for continuous variables, medians (minimum–maximum) for ordinal ones, and frequency with percentages for categorical ones. The Kolmogorov–Smirnov test was used to analyse the distribution of data. Differences in measured parameters between the two groups were analysed using independent-samples t-tests or Mann–Whitney U-tests, as appropriate. Categorical comparisons were made using χ2 or Fisher's exact tests, as appropriate. A p-value <0.05 was considered to indicate statistical significance.
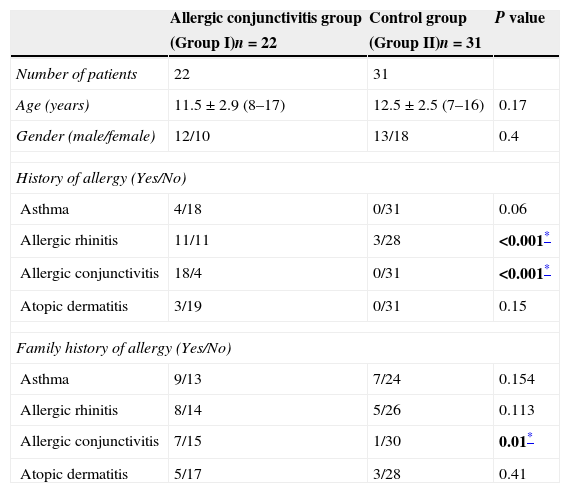
ResultsThe allergic rhinoconjunctivitis group (Group I; n=22) included 12 male and 10 female children whose mean age was 11.5±2.9 years. The healthy control group (Group II; n=31), included 13 male and 18 female subjects whose mean age was 12.5±2.5 years. There was no difference between Groups I and II in terms of age or gender (p=0.17 and 0.4, respectively).
Histories of allergic rhinitis and allergic conjunctivitis were significantly more common in Group I than in Group II (both p<0.001). Family histories of asthma, allergic rhinitis, and atopic dermatitis did not differ significantly between the groups (all p>0.05). However, family history of allergic conjunctivitis was significantly higher in Group I than in Group II (p=0.01). Demographics and clinical characteristics of the groups are summarised in Table 1.
Demographic and clinical characteristics of the groups.
| Allergic conjunctivitis group | Control group | P value | |
|---|---|---|---|
| (Group I)n=22 | (Group II)n=31 | ||
| Number of patients | 22 | 31 | |
| Age (years) | 11.5±2.9 (8–17) | 12.5±2.5 (7–16) | 0.17 |
| Gender (male/female) | 12/10 | 13/18 | 0.4 |
| History of allergy (Yes/No) | |||
| Asthma | 4/18 | 0/31 | 0.06 |
| Allergic rhinitis | 11/11 | 3/28 | <0.001* |
| Allergic conjunctivitis | 18/4 | 0/31 | <0.001* |
| Atopic dermatitis | 3/19 | 0/31 | 0.15 |
| Family history of allergy (Yes/No) | |||
| Asthma | 9/13 | 7/24 | 0.154 |
| Allergic rhinitis | 8/14 | 5/26 | 0.113 |
| Allergic conjunctivitis | 7/15 | 1/30 | 0.01* |
| Atopic dermatitis | 5/17 | 3/28 | 0.41 |
The mean serum IgE concentration in Group I was 143.6±132.8IU/ml, and that in Group II was 54.8±44.1IU/ml. The serum IgE concentration in Group I was significantly higher than that in Group II (p=0.03).
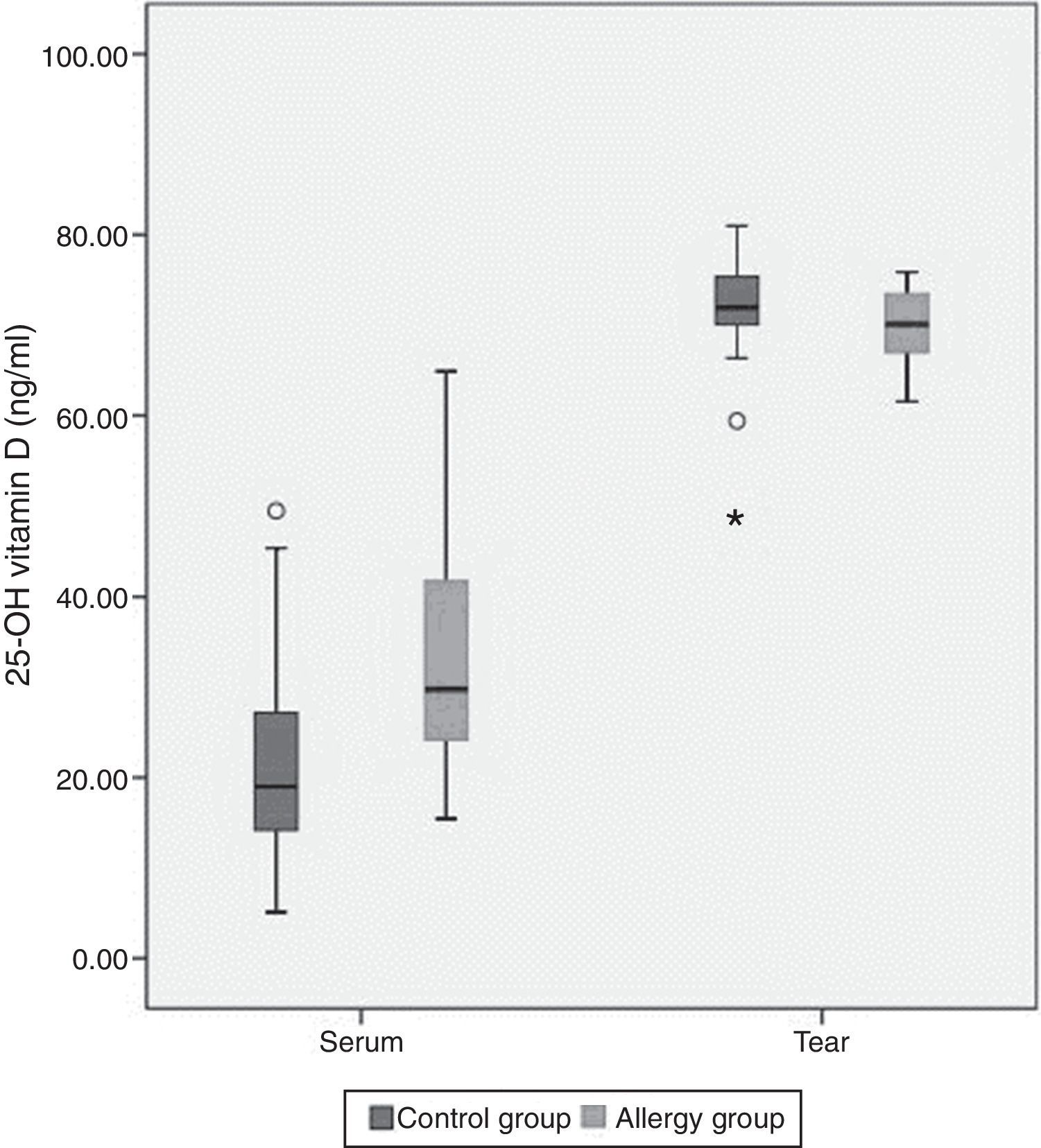
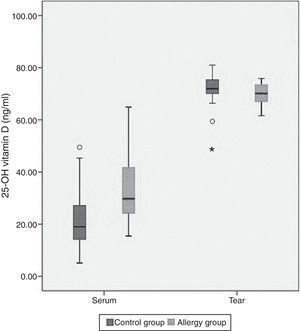
The mean tear and serum 25(OH)D levels in Group I were 70±3.9ng/ml and 34.1±12.7ng/ml, and those in Group II were 71.8±6.2ng/ml and 21.8±11.3ng/ml, respectively (Table 2). Serum 25(OH)D levels were significantly higher in children with allergic rhinoconjunctivitis than in healthy controls (p=0.001), whereas tear levels of 25(OH)D did not differ significantly between the groups (p=0.25). Tear levels of 25(OH)D were significantly higher compared with serum levels in both groups (p<0.001). However, there was no significant correlation between serum and tear 25(OH)D levels. Fig. 1 shows the serum and tear 25(OH)D levels of the groups in a clustered box plot graph.
Tear and serum vitamin D levels and serum total IgE levels of both groups.
This is the first report investigating the relationship between allergic conjunctivitis and serum and tear fluid vitamin D status in children. Also, our study has a unique feature in that we evaluated vitamin D levels in the tear fluid of humans. Plasma levels of vitamin D in patients with allergic rhinoconjunctivitis were significantly higher than those in the control group. Additionally, tear fluid vitamin D levels were significantly higher than plasma levels in both groups.
Vitamin D is a prohormone that is synthesised in the skin after exposure to ultraviolet radiation, and it plays an important role in calcium homeostasis and bone health. It also serves as a hormone, as it is involved in cardiovascular diseases, cancer, obesity, immune function, and maternal–foetal health.10 In addition to its role in the regulation of calcium metabolism, vitamin D has a number of immunological effects.11
We found that serum vitamin D levels were significantly higher in children with allergic rhinoconjunctivitis than in healthy controls, whereas tear levels of vitamin D did not differ significantly between the groups. Tear levels of vitamin D were significantly higher than serum levels in both groups. This may be due to a self-control or barrier mechanism in the anterior segment of the eye.12
The literature regarding the relationship between vitamin D and allergic diseases is inconsistent. It is possible that vitamin D3 intake during infancy and in the prenatal period may play a role in the epidemiology of allergic diseases.6 In a prospective birth cohort study, Hyppönen showed that vitamin D supplementation in infancy caused an increased risk of atopy and allergic rhinitis in later years of life 6,7; that study also found a significant association between a high vitamin D3 intake during infancy and atopic dermatitis in later childhood.6 Another study revealed that early infant multivitamin supplementation was associated with an increased risk of asthma and food allergy.13
In contrast to the studies mentioned above, several recent studies suggested that vitamin D deficiency is associated with an increased risk for allergic diseases. Wang et al. showed that serum vitamin D levels were inversely correlated with atopic dermatitis severity.14 A cohort study found that the risk for wheezing and eczema were significantly decreased among children 16–24 months of age whose mothers had had higher vitamin D intakes during pregnancy.5 In a birth cohort study, the Prevention and Incidence of Asthma and Mite Allergy (PIAMA) study, serum vitamin D concentrations at age four years were inversely associated with asthma at ages 4–8 years.15 It was also shown that allergic sensitisation to several allergens was more common in children with 25(OH)D deficiency.16 Moreover, Rothers et al.17 found that both low and high cord blood levels of vitamin D were associated with increased total IgE and inhalant allergen-specific IgE levels and with a positive skin prick test.
Some animal studies have also reported seemingly paradoxical results regarding the relationship between vitamin D supplementation and allergies. Matheu et al. reported that early treatment with vitamin D enhanced allergen-induced T-cell proliferation as well as Th2 cytokines (IL-4 and IL-13) and IgE production. Paradoxically, recruitment of eosinophils and IL-5 levels were impaired in lung tissue, and this improved the local inflammatory response. They suggested that excessive supplementation of vitamin D could influence the development of a sustained Th2 response, leading to an increased prevalence of allergy, whereas vitamin D might hold promising beneficial effects in airway eosinophilia.18 These conflicting results suggest that not only low levels but also high levels of vitamin D may play a role in the pathogenesis of allergic diseases, which may be related to its immune modulatory effects.
To our knowledge, this is the first reported study to evaluate the vitamin D level in tear fluids in humans. In an animal study, investigators found measurable concentrations of vitamin D metabolites in tear fluid, and oral vitamin D supplementation affected vitamin D metabolite concentrations in the anterior segment of the eye in rabbits.12 Additionally, the corneal limbal epithelium is capable of synthesising 25(OH)D3 following UV-B exposure. They also measured different metabolites of vitamin D and showed that 25(OH)D3 was elevated in tear and aqueous humour following vitamin D3 supplementation. As in our study, they found that the vitamin D concentration in tear fluid of rabbits was higher than vitamin D levels in plasma.12
This study has several strengths. First, it is a novel study that investigated association between vitamin D status and allergic conjunctivitis in children. Second, this is the first study to determine levels of vitamin D in human tear fluid. Furthermore, we collected the samples in the same season to exclude variations in vitamin D levels due to sun exposure.
The study also has several limitations. First, due to difficulties in taking tear fluid samples from the eyes especially in children, the number of patients was low. Second, the study was limited by having only one measure of vitamin D status. Third, skin tests or serum specific IgE tests could not be performed.
In conclusion, our study suggests that high levels of vitamin D may have a role in ARC, but the mechanisms behind these findings remain unclear. Further study is required to determine that vitamin D supplementation in infancy and pregnancy may have long-term effects on allergy development.
Conflict of interestNone of the authors have conflict of interest.
Ethical disclosuresPatients’ data protectionConfidentiality of Data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
This study was supported by the Research Fund of the Abant Izzet Baysal University, Turkey (Project no: 2013.08.03.679).