The pathogenesis of exercise-induced bronchoconstriction (EIB) in asthma is incompletely understood. The role of exhaled breath condensate (EBC) annexin A5, which is an anti-inflammatory mediator, has not been investigated. The purpose of this study is to evaluate EBC annexin A5 levels in EIB in asthmatic children.
MethodsTwo groups of children were enrolled in this study: asthmatic children with positive (n=11) and negative (n=7) responses to exercise. The levels of pre- and post-exercise EBC annexin A5 were determined with using enzyme-linked immunosorbent assay (ELISA).
ResultsWe observed significant higher pre-exercise EBC annexin A5 levels in the challenge test negative children than in the challenge test positive children (p<0.05). No significant difference was observed in the post-exercise EBC annexin A5 levels between the groups (p>0.05). Also, no significant difference was observed between pre- and post-exercise EBC annexin A5 levels within each group (p>0.05). There was an inverse correlation between annexin A5 levels and a reduction in forced expiratory volume at one second percent (FEV1%) (p=0.009, r=−0.598).
ConclusionsOur preliminary study showed that EBC annexin A5 may have a possible preventive role in EIB in asthma. Annexin A5 and related compounds may provide novel therapeutic approaches to the treatment of EIB in asthma.
Asthma is a chronic disease characterized by airway inflammation, episodic symptoms, and airflow obstruction. Chronic inflammation observed in asthma leads to airway hyperresponsiveness (AHR) which is defined as an abnormal increase in airflow limitation following exposure to a non-allergic stimulus such as exercise.1–3 Exercise-induced bronchoconstriction (EIB) can be demonstrated in up to 70% of patients with asthma. However, the reasons for EIB are not clear in patients with asthma. It has been suggested that exercise causes airway narrowing due to the loss of water through evaporation, resulting in thermal and osmotic effects of dehydration in the airways. However, the precise mechanism by which increased osmolarity leads to airflow limitation is unknown.4–6 It has been proposed that mediators, such as histamine, leukotrienes, and prostanoids, released from the mast cells and epithelial cells in response to a hyperosmolar stimulus may be responsible for the bronchospasm.7 Annexins belong to the family of Ca2+ dependent membrane binding proteins associated with membrane related processes.8 They are involved in several cellular activities such as membrane trafficking, exocytosis, endocytosis, membrane–cytoskeleton interactions, as an anticoagulant, and anti-inflammatory protein, among others.9,10 Annexin A5, which is located in extracellular and intracellular milieu, shows anti-inflammatory, anti-coagulant and anti-apoptotic effects through binding expressed on the plasma membrane phosphatidylserine.11,12 In this study, based on the anti-inflammatory properties of annexin A5, we investigated exhaled breath condensate (EBC) annexin A5 level before and after exercise challenge in children with asthma.
Material and methodsAll study procedures were carried out in accordance with a protocol previously approved by the Institutional Review Board of Erciyes University. Written inform consent was obtained from all patients before the procedure and all children gave assent.
Study populationChildren with asthma aged 5–17 years were included from the Allergy and Asthma Unit of Erciyes University, School of Medicine, Kayseri, Turkey. Asthma was diagnosed according to the published GINA guidelines.13 In an attempt to prevent the severity of disease from being a confounding factor, only children who strictly fulfilled the criteria for mild asthma were enrolled and those with moderate to severe asthma were excluded from the study.13 Children who had an upper or lower airway infection or had had an asthma exacerbation within the previous six weeks were excluded from the study. All children underwent a standard exercise challenge with treadmill. EBC was collected before and immediately after the exercise challenge. Spirometric measurements, total IgE, and eosinophil counts were obtained and skin prick testing (Allergopharma, Reinbek, Germany) was done with 21 antigens including 13 aero-allergens (Dermatophagoides farinae, Dermatophagoides pteronyssinus, Alternaria, Aspergillus, Cladosporium, Saccharomyces, cat dander, grass mix 12, Betulaceae, Salicaceae, Compositae, grasses, and cockroach) and eight food allergens (cow milk, egg white, egg yolk, soy, nut, peanut, wheat, and tuna fish) with appropriate positive and negative controls on the upper back of the children at presentation. Reactions were accepted as positive if an induration bigger than >3mm was observed according to the negative control.
Collection of EBCEBC was collected using a commercial device (R tube; Respiratory Research, Inc., Charlottesville, VA, USA), as previously described, and following the guidelines of the European Respiratory Society.14 Subjects were instructed to breathe tidally for 10min through the mouthpiece of the R tube with a one-way valve, which was connected to a condenser. Subjects were also instructed to temporarily discontinue EBC collection if they needed to swallow saliva or had an urge to cough. Also, in addition to instruction, a saliva trap was mounted to R tube to avoid salivary contamination. The EBC samples were transferred into sterile containers and immediately stored at −70°C until analysis.
Study designExercise testing was conducted according to a previously described protocol. Short-acting bronchodilators were discontinued for at least 12h (hour), long-acting bronchodilators for at least 48h, antihistamines for at least one week, and leukotriene modifiers for at least 72h in accordance with American Thoracic Society guidelines.15 Children who had used inhaled corticosteroids within the previous two weeks were excluded from the study. Children with asthma increased their exercise effort until their heart rate reached 85% of the maximum for their predicted age group within one minute of starting the test and maintained it during 6min of exercise. Jaeger 2004 spirometer was used for spirometric evaluations. FEV1 was measured at 1, 5, 10, 15, and 20minutes after exercise. Prior to spirometry, the required manoeuvres were demonstrated by a respiratory technician. The required manoeuvres included tidal breathing for a while followed by a maximal inspiration and maximal expiration. Measurements were performed at least three times in subjects with an acceptable technique in a sitting position and wearing nose clips. The highest level for FVC% (forced vital capacity), FEV1% (forced expiratory volume at one second percent), FEF 25–75% (forced expiratory flow between 75% and 25% of vital capacity), and PEF% (peak expiratory flow) was evaluated. A reduction in FEV1 of at least 10% of the pre-exercise value was considered positive. Reference for predicted FEV1 (%) value was accepted as 80% and over. IgE levels were measured with the nephelometric method and eosinophil counts were determined from coulter counter leucocyte measurements.
Annexin A5 measurementsThe ELISA kit was used to detect human annexin A5 levels (EBioscience Company, BMS252/BMS252TEN, USA), according to the manufacturer's instructions. Briefly, the microwells were coated with the anti-human annexin V coating antibody. 100μl of sample diluent was added in duplicate to the blank wells, 50μl of sample diluent was added to the sample wells, and 50μl of each plasma sample in duplicate was added to the sample wells. Subsequently, 50μl of biotin-conjugate was added to all wells and was incubated at room temperature (RT) for 2h. After the incubation the microwell strips were washed four times. 100μl of diluted Streptavidin-HRP was added to all wells and was incubated at RT for 1h. 100μl of TMB Substrate Solution was added to all wells following the four times washing and was incubated at RT for 10min for the colour development. Later, 100μl stop solution was added to all wells and the plate was read on ELISA reader at 450nm. A standard curve was prepared from seven human annexin V standard dilutions and human annexin V sample concentration determined.
Statistical analysesShapiro–Wilk test was carried out to determine normality of data distribution. Shapiro–Wilk test revealed abnormal data distribution for values of annexin A5, total IgE, eosinophil counts, baseline predicted FEV1%, and post-exercise maximal decrease in FEV1% (p<0.05). Because of the abnormal data distribution of these values, median values (Interquartile range) were determined and compared with Mann–Whitney U test between groups. The comparison of the percentage of atopy positivity and gender between groups were performed with Chi-square test between groups. For age, height, and body weight values, mean values were determined and compared with independent t test because of normal data distribution between groups (p>0.05). Annexin A5 levels in EBC were compared by Wilcoxon W test within each group. Correlation analyses were evaluated with Spearman correlation test. A p value of less than 0.05 was considered significant.
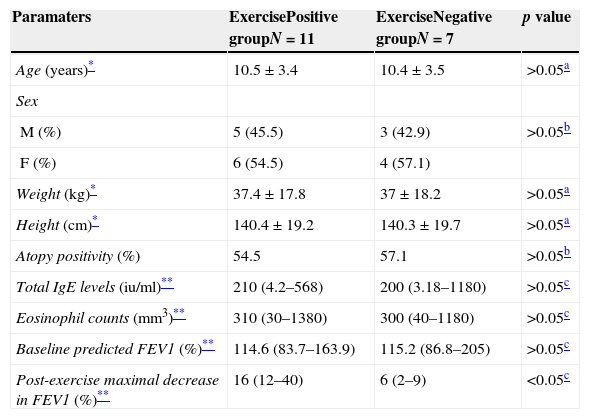
ResultsPatients characteristicsA total of 18 children with asthma were screened for exercise response. Eleven children with a positive exercise response (exercise response positive asthmatics) and seven children with a negative response (exercise response negative asthmatics) were included in the study. There were no significant differences between the two groups with respect to age, gender, height, weight, atopy positivity, total IgE levels, eosinophil counts, and baseline predicted FEV1% values (p>0.05, Table 1).
Patients’ characteristics.
| Paramaters | ExercisePositive groupN=11 | ExerciseNegative groupN=7 | p value |
|---|---|---|---|
| Age (years)* | 10.5±3.4 | 10.4±3.5 | >0.05a |
| Sex | |||
| M (%) | 5 (45.5) | 3 (42.9) | >0.05b |
| F (%) | 6 (54.5) | 4 (57.1) | |
| Weight (kg)* | 37.4±17.8 | 37±18.2 | >0.05a |
| Height (cm)* | 140.4±19.2 | 140.3±19.7 | >0.05a |
| Atopy positivity (%) | 54.5 | 57.1 | >0.05b |
| Total IgE levels (iu/ml)** | 210 (4.2–568) | 200 (3.18–1180) | >0.05c |
| Eosinophil counts (mm3)** | 310 (30–1380) | 300 (40–1180) | >0.05c |
| Baseline predicted FEV1 (%)** | 114.6 (83.7–163.9) | 115.2 (86.8–205) | >0.05c |
| Post-exercise maximal decrease in FEV1 (%)** | 16 (12–40) | 6 (2–9) | <0.05c |
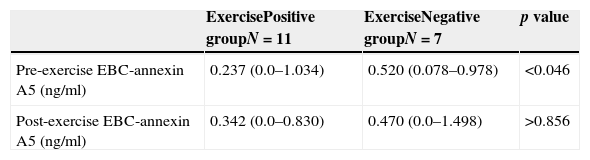
We observed significant higher pre-exercise EBC annexin A5 levels in the challenge test negative children than in the challenge test positive children (p<0.05, Mann–Whitney U, Table 2). No significant difference was observed in the post-exercise EBC annexin A5 levels between the groups (p>0.05, Mann–Whitney U, Table 2). Also, no significant difference was observed between pre- and post-exercise EBC annexin A5 levels within each group (p>0.05, Wilcoxon).
EBC annexin A5 levels (ng/ml).
| ExercisePositive groupN=11 | ExerciseNegative groupN=7 | p value | |
|---|---|---|---|
| Pre-exercise EBC-annexin A5 (ng/ml) | 0.237 (0.0–1.034) | 0.520 (0.078–0.978) | <0.046 |
| Post-exercise EBC-annexin A5 (ng/ml) | 0.342 (0.0–0.830) | 0.470 (0.0–1.498) | >0.856 |
Within each group, there was no significant difference between pre- and post-exercise EBC annexin A5 (p>0.05, Wilcoxon test).
We could not find any significant correlation between EBC annexin A5 levels with eosinophil counts and total IgE levels (p>0.05, Spearman). There was an inverse correlation between annexin A5 levels and a reduction in post-exercise FEV1% (p=0.009, r=−0.598, Spearman).
DiscussionTo our knowledge, this is the first study to investigate the role of EBC annexin A5 in EIB in asthmatic patients. Our results displayed significant higher pre-exercise EBC annexin A5 levels in the challenge test negative children than in the exercise challenge test positive children. Also, an inverse correlation was observed between annexin A5 levels and a reduction in post-exercise FEV1% values. It has been accepted that EIB is a prototypical manifestation of indirect AHR which is one of the manifestations of airway inflammation. Airway inflammation in asthmatic patients is characterized by upregulation of multiple inflammatory mediators and cytokines. Arachidonic acid-derived leukotrienes are the most potent inflammatory mediators.16,17 Anti-inflammatory membrane lipid mediators are generated during airway inflammation processes to promote inflammatory resolution. Defects in the production of the anti-inflammatory lipid mediators have been reported associated with persistent airway inflammation. The most known anti-inflammatory mediators are lipoxins. It was reported that EIB negative asthmatic patients have significant higher levels of the protective lipoxin A4 than EIB positive asthmatic patients.18 In another study reported by Eke Gungor et al.,19 wheezy infants have significantly lower levels of the lipoxin A4 and annexin A1 than controls. In addition to lipoxins, recently several new fatty acid-derived anti-inflammatory mediators have been discovered including resolvins, protectins, and mareins.20,21 Annexins are a family of calcium-dependent membrane binding proteins that share structural properties and biological activities associated with membrane related processes. One of the best-known processes regulated by annexins is inflammation. Some annexins such as annexin A1, A2, and A4 contribute to the pathophysiology of asthma. In particular, annexin A1 is able to inhibit different enzymes involved in the inflammatory process, including phospholipase A2 (PLA2), cyclooxygenase-2 and inducible nitric oxide synthase.22 Ng et al.23 stated that annexin A1 (−/−) mice possess multiple features characteristic to allergic asthma, such as AHR and increased antibody responses, suggesting that annexin A1 plays a critical regulatory role in the development of asthma. Plasminogen also has a role in the airway inflammation. Airway smooth muscle cells convert plasminogen to plasmin which is regulated by annexin A2. Plasmin is a protease which has pro-inflammatory activities.24 Park et al.25 reported that annexin A4 polymorphism could have susceptibility for aspirin-exacerbated respiratory disease. With regard to annexin A5, it has anti-inflammatory features as well as strong anticoagulant and antithrombotic properties.26 Annexin A5 inhibits pro-inflammatory cytokines such as TNF-α and IL-1β expression.27 Also, annexin A5 inhibits secretory phospholipase A2 (sPHA2).28 In an experimental study, it was demonstrated that sPLA2 (−/−) mice have markedly decreased parameters of Th2-driven airway inflammation and remodelling than sPLA2 (+/+) mice. It was reported that sPHA2 is expressed in the airway epithelial cells and macrophages in bronchoalveolar lavage fluid.29 In the medical literature the association of annexin A5 and asthma has not been investigated to date. In this study, based on the anti-inflammatory properties of annexin A5, we investigated EBC annexin A5 levels before and after exercise challenge in children with asthma. We observed significantly higher pre-exercise EBC annexin A5 levels in the challenge test negative children than in the challenge test positive children and an inverse correlation was observed between annexin A5 levels and a reduction in post-exercise FEV1% levels.
As a result, we observed that annexin A5 may have a preventive role in EIB which is related to the anti-inflammatory features in children with asthma. However, the number of participants in the presented study was too small to make a decision. Further studies with more participants are needed to make a decision on this topic. If more supportive results can be found between annexin A5 and inflammation in the airways of asthmatic patients, annexin A5 and related compounds may provide novel therapeutic approaches for the treatment of EIB in asthmatic patients.
Conflict of interestThe authors declare that they have no conflict of interest.
Ethical disclosuresPatients’ data protectionThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.