To determine the prevalence of wheezing during the first year of life in Cantabria, Spain and its associated risk factors.
MethodologyA cross-sectional, multicentre, descriptive epidemiological study was carried out in a representative sample of 958 infants in the first year of life, born in Cantabria. A previously validated and standardised written questionnaire was completed by the parents of infants seen between 12 and 15 months of age in the Primary Care Centres.
ResultsThe prevalence of wheezing was 32.7%. A relationship was found with male gender (OR 1.38, 95%CI [1.05–1.81]), the presence of a sibling (OR 2.43 [1.38–3.98]), attending nursery school (OR 2.40 [1.71–3.35]), exclusive breastfeeding for <3 months (OR 1.47 [1.12–1.93]), a first cold at ≤3 months (OR 2.07 [1.56–2.74]), asthma in siblings (OR 2.17 [1.25–3.77]), parental allergic rhinitis (OR 1.62 [1.10–2.37]) and paracetamol use >1 a week (OR 2.49 [1.31–4.73]), and maternal smoking during pregnancy (OR 2.18 [1.51–3.15]). The prevalence of recurrent wheezing (≥3 episodes) was 14.3%. Significant associations were observed with the male gender (OR 1.79 [1.23–2.60]), attending nursery school (OR 2.92 [1.96–4.35]), first cold at ≤3 months (OR 2.11 [1.46–3.04]), eczema (OR 1.92 [1.21–3.04]), maternal asthma (OR 1.77 [1.00–3.14]), exclusive breastfeeding for <3 months (OR 1.53 [1.06–2.22]), and maternal smoking during pregnancy (OR 1.53 [1.05–2.22]).
ConclusionsOne third of the infants experienced wheezing during the first year of life; those who were less exclusively breastfed, attended nursery school, presented eczema, family asthma or allergic rhinitis, and maternal smoking during pregnancy.
Wheezing is one of the most frequent causes of consultation in paediatrics and of hospital admission in the first years of life. Recurrent wheezing (RW) has a significant impact upon the quality of life of the patients and their families,1 and generates an important consumption of healthcare resources and economic costs.
Using a simple and standardised methodology based on an epidemiological survey, phases I–III of the International Study of Asthma and Allergies in Childhood (ISAAC) has produced the first large-scale body of data on asthma in children and its associated risk factors. This has made it possible to establish comparisons among different countries and centres in relation to children between 6 and 7 years of age and adolescents between 13 and 14 years of age.2
However, although wheezing is a worldwide public health problem, and different studies involving cohorts investigated from birth have shed considerable light on the origin of wheezing in the first years of life,3,4 there have been few international studies on the prevalence of wheezing in nursing infants and of the risk factors capable of accounting for wheezing in this particular age population. As a result, in recent years a number of studies have addressed this specific age group, focusing on viral causes,5 allergic influences,6,7 obstetric antecedents,8 early exposure to certain allergens, environmental exposures such as smoking,9 pollution and pets,10 the use of certain drug substances11 or the consumption of different foods during pregnancy.12
In this context, and considering the experience of the ISAAC in children and adolescents, large multicentre studies using simple and standardised methodology are needed to determine and compare the prevalence and severity of wheezing in the nursing infant during the first year of life, and to investigate its association to certain risk factors. Such studies in turn will help pave the way for future investigations on the aetiology and evolution of the prevalence of wheezing in relation to genetic and environmental factors, life styles and medical care.
The present study has been carried out following the standardised method of the International Study of Wheezing in Infants (Estudio Internacional de Sibilancias en Lactantes, EISL), involving a questionnaire applied to a very large sample of infants under one year of age in Latin America, Spain and The Netherlands.13
Materials and methodsThe study was carried out using the EISL questionnaire in its validated Spanish version,14 involving methodology based on that employed in phases I and III of the ISAAC among older children.2 The present study forms part of the EISL conducted in Cantabria, Spain, and the questionnaire includes questions on wheezing in the first year of life and on the possible associated risk and/or protective factors.
Study designA cross-sectional, multicentre, descriptive epidemiological study was carried out in a representative sample of 958 infants in the first year of life, born in the Community of Cantabria, and who visited their Primary Care Centre between 1 June 2008 and 31 March 2011.
Study subjectsThe questionnaire was administered to the parents of the infants visiting the Primary Care Centres in Cantabria (both rural and urban) on occasion of the scheduled health check-up between 12 and 15 months of age. Infants with parents who failed to complete the questionnaire or who produced an incomplete or incorrect questionnaire were excluded from the study, as were those infants in which the number of wheezing episodes in the first year of life could not be established, and those cases in which written informed consent was not obtained.
Study variablesThe primary study variable was the presence or absence of wheezing during the first year of life. An infant was considered to have wheezing when a positive reply was obtained to the question: “Has your child experienced wheezing or whistling sounds in the chest in the first 12 months of life?” Recurrent wheezing (RW) was defined as three or more episodes of wheezing during the first year of life. In turn, severe wheezing (SW) was defined by a positive reply to the question: “In the first 12 months of life has your child experienced wheezing or whistling sounds in the chest that were so intense that they produced suffocation or great breathing difficulties?” In addition, we recorded a series of variables related to wheezing, such as the number of episodes; patient age at onset; association to exercise, laughing or crying; influence of wheezing upon eating or sleeping in the infant or on the activities of the parents; impact of wheezing in terms of changes in family life; treatments received; emergency care visits; diagnosis of asthma, relation to eczema; and severity. Furthermore, we analysed variables related to determinants or risk and/or protective factors such as gender; weight and height at birth and at one year of age; race or ethnicity; place of birth; maternal smoking during pregnancy and smoking in the mother or other relatives after birth; family history of asthma, rhinitis, eczema or allergic diseases; exclusive breastfeeding; infant age at first cold; attending nursery school; presence of pets in the home at the time of birth of the infant and during the first year of life; educational level of the parents; conditions in the home (e.g., presence of mould or carpets); and maternal eating habits during pregnancy (“fast food”, Mediterranean diet).
Statistical analysisThe completed questionnaires were scanned (Fujitsu M4097D) using the optical Mark Recognition program Remark Office OMR v6 (Principia products, Paoli, PA, USA).
Frequencies and percentages were calculated for the descriptive study of qualitative variables, with the determination of means and standard errors in the case of quantitative variables. The chi-squared test was used to evaluate associations between qualitative variables, whilst the Student's t-test or analysis of variance (ANOVA) was used to establish relationships between qualitative and quantitative variables, as applicable. A Microsoft Excel 2013 application was used to calculate the odds ratios (ORs) with their corresponding confidence intervals and p-values. Statistical significance was considered for p<0.05. The SPSS version 16 statistical package was used.
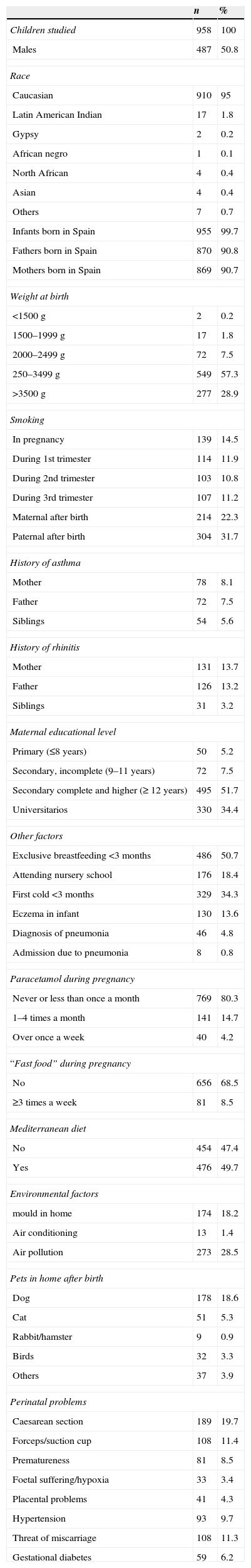
ResultsA total of 958 infants were studied (of whom 487 were males, 50.8%), with a body weight of 2500–3499g in 57.3% of the cases, and a mean height at birth of 50.09cm (standard error 2.43). The descriptive data of the study sample are shown in Table 1.
Study subjects. Descriptive results.
| n | % | |
|---|---|---|
| Children studied | 958 | 100 |
| Males | 487 | 50.8 |
| Race | ||
| Caucasian | 910 | 95 |
| Latin American Indian | 17 | 1.8 |
| Gypsy | 2 | 0.2 |
| African negro | 1 | 0.1 |
| North African | 4 | 0.4 |
| Asian | 4 | 0.4 |
| Others | 7 | 0.7 |
| Infants born in Spain | 955 | 99.7 |
| Fathers born in Spain | 870 | 90.8 |
| Mothers born in Spain | 869 | 90.7 |
| Weight at birth | ||
| <1500g | 2 | 0.2 |
| 1500–1999g | 17 | 1.8 |
| 2000–2499g | 72 | 7.5 |
| 250–3499g | 549 | 57.3 |
| >3500g | 277 | 28.9 |
| Smoking | ||
| In pregnancy | 139 | 14.5 |
| During 1st trimester | 114 | 11.9 |
| During 2nd trimester | 103 | 10.8 |
| During 3rd trimester | 107 | 11.2 |
| Maternal after birth | 214 | 22.3 |
| Paternal after birth | 304 | 31.7 |
| History of asthma | ||
| Mother | 78 | 8.1 |
| Father | 72 | 7.5 |
| Siblings | 54 | 5.6 |
| History of rhinitis | ||
| Mother | 131 | 13.7 |
| Father | 126 | 13.2 |
| Siblings | 31 | 3.2 |
| Maternal educational level | ||
| Primary (≤8 years) | 50 | 5.2 |
| Secondary, incomplete (9–11 years) | 72 | 7.5 |
| Secondary complete and higher (≥ 12 years) | 495 | 51.7 |
| Universitarios | 330 | 34.4 |
| Other factors | ||
| Exclusive breastfeeding <3 months | 486 | 50.7 |
| Attending nursery school | 176 | 18.4 |
| First cold <3 months | 329 | 34.3 |
| Eczema in infant | 130 | 13.6 |
| Diagnosis of pneumonia | 46 | 4.8 |
| Admission due to pneumonia | 8 | 0.8 |
| Paracetamol during pregnancy | ||
| Never or less than once a month | 769 | 80.3 |
| 1–4 times a month | 141 | 14.7 |
| Over once a week | 40 | 4.2 |
| “Fast food” during pregnancy | ||
| No | 656 | 68.5 |
| ≥3 times a week | 81 | 8.5 |
| Mediterranean diet | ||
| No | 454 | 47.4 |
| Yes | 476 | 49.7 |
| Environmental factors | ||
| mould in home | 174 | 18.2 |
| Air conditioning | 13 | 1.4 |
| Air pollution | 273 | 28.5 |
| Pets in home after birth | ||
| Dog | 178 | 18.6 |
| Cat | 51 | 5.3 |
| Rabbit/hamster | 9 | 0.9 |
| Birds | 32 | 3.3 |
| Others | 37 | 3.9 |
| Perinatal problems | ||
| Caesarean section | 189 | 19.7 |
| Forceps/suction cup | 108 | 11.4 |
| Prematureness | 81 | 8.5 |
| Foetal suffering/hypoxia | 33 | 3.4 |
| Placental problems | 41 | 4.3 |
| Hypertension | 93 | 9.7 |
| Threat of miscarriage | 108 | 11.3 |
| Gestational diabetes | 59 | 6.2 |
The prevalence of wheezing was 32.7%. Of the 313 infants with wheezing in the first year of life, 99 suffered a single episode; 77 presented two episodes; and 137 infants had three or more episodes. Thus, the prevalence of RW was 14.3%. Only 94 of the 313 infants with wheezing suffered SW, but according to the parents, the prevalence of SW in the first year of life was 9.8% of the total sample.
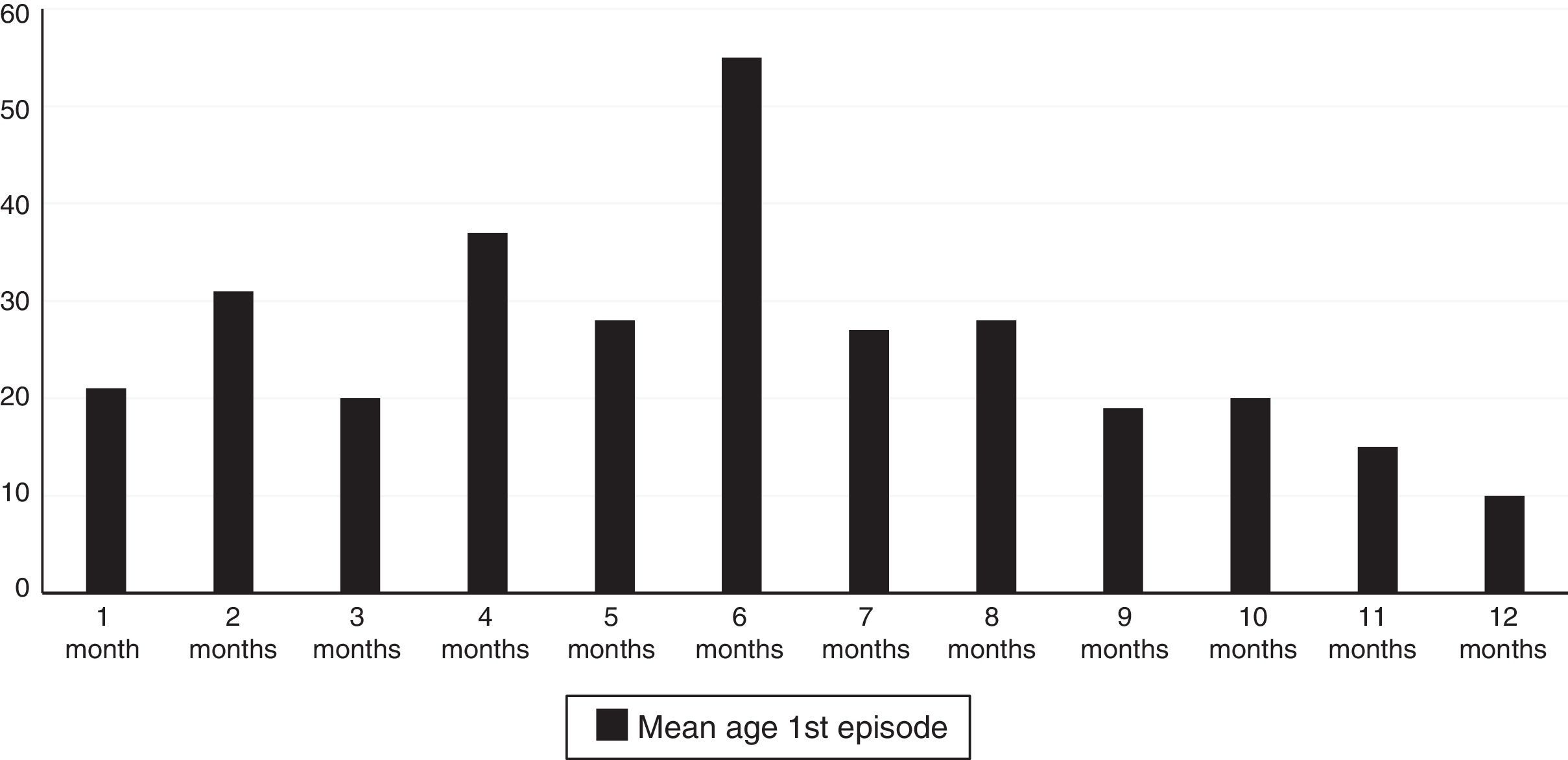
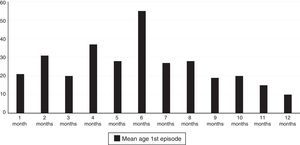
A total of 61.3% of the infants with wheezing developed the condition in the first six months of life, and 78.9% did so in the first eight months. The mean age at the time of the first episode was 5.88 months (standard error 2.97), and the age interval in which the appearance of the first wheezing episode proved most frequent was between 4 and 6 months (Fig. 1).
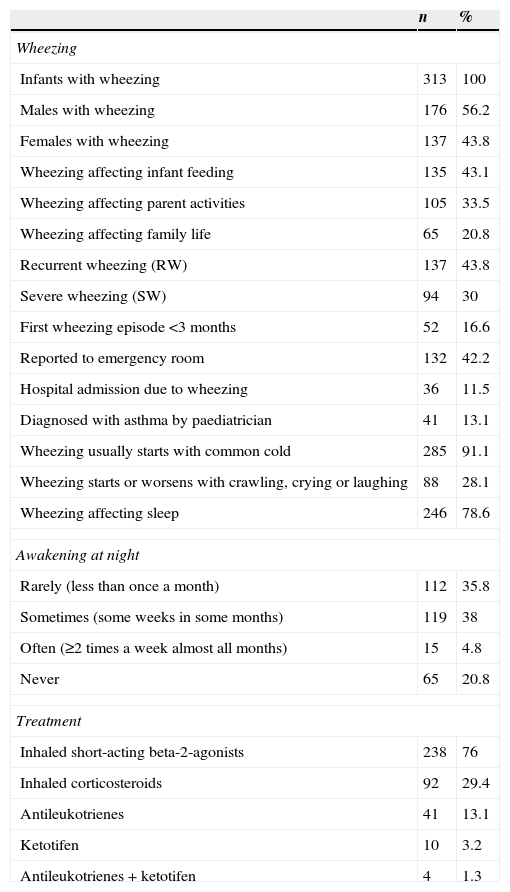
The descriptive data corresponding to the infants with wheezing are shown in Table 2. Of the 313 infants with wheezing, 132 (42.2%) required emergency care, and 36 (11.5%) had to be hospitalised.
Descriptive results in the infants who have presented wheezing.
| n | % | |
|---|---|---|
| Wheezing | ||
| Infants with wheezing | 313 | 100 |
| Males with wheezing | 176 | 56.2 |
| Females with wheezing | 137 | 43.8 |
| Wheezing affecting infant feeding | 135 | 43.1 |
| Wheezing affecting parent activities | 105 | 33.5 |
| Wheezing affecting family life | 65 | 20.8 |
| Recurrent wheezing (RW) | 137 | 43.8 |
| Severe wheezing (SW) | 94 | 30 |
| First wheezing episode <3 months | 52 | 16.6 |
| Reported to emergency room | 132 | 42.2 |
| Hospital admission due to wheezing | 36 | 11.5 |
| Diagnosed with asthma by paediatrician | 41 | 13.1 |
| Wheezing usually starts with common cold | 285 | 91.1 |
| Wheezing starts or worsens with crawling, crying or laughing | 88 | 28.1 |
| Wheezing affecting sleep | 246 | 78.6 |
| Awakening at night | ||
| Rarely (less than once a month) | 112 | 35.8 |
| Sometimes (some weeks in some months) | 119 | 38 |
| Often (≥2 times a week almost all months) | 15 | 4.8 |
| Never | 65 | 20.8 |
| Treatment | ||
| Inhaled short-acting beta-2-agonists | 238 | 76 |
| Inhaled corticosteroids | 92 | 29.4 |
| Antileukotrienes | 41 | 13.1 |
| Ketotifen | 10 | 3.2 |
| Antileukotrienes+ketotifen | 4 | 1.3 |
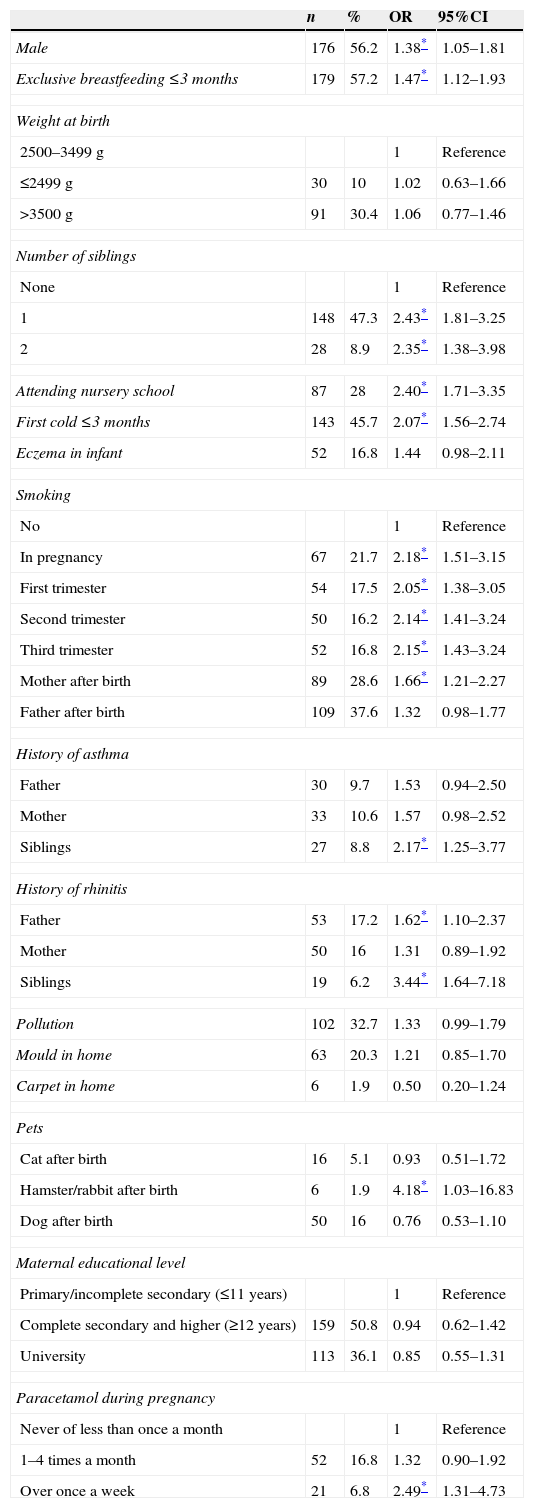
Tables 3 and 4 in turn show the results of the associations between the different study variables and wheezing and RW during the first year of life. The ORs and 95% confidence intervals (95%CIs) have been calculated.
Wheezing risk factors. Odds ratio (OR) with 95% confidence interval (95%CI).
| n | % | OR | 95%CI | |
|---|---|---|---|---|
| Male | 176 | 56.2 | 1.38* | 1.05–1.81 |
| Exclusive breastfeeding ≤3 months | 179 | 57.2 | 1.47* | 1.12–1.93 |
| Weight at birth | ||||
| 2500–3499g | 1 | Reference | ||
| ≤2499g | 30 | 10 | 1.02 | 0.63–1.66 |
| >3500g | 91 | 30.4 | 1.06 | 0.77–1.46 |
| Number of siblings | ||||
| None | 1 | Reference | ||
| 1 | 148 | 47.3 | 2.43* | 1.81–3.25 |
| 2 | 28 | 8.9 | 2.35* | 1.38–3.98 |
| Attending nursery school | 87 | 28 | 2.40* | 1.71–3.35 |
| First cold ≤3 months | 143 | 45.7 | 2.07* | 1.56–2.74 |
| Eczema in infant | 52 | 16.8 | 1.44 | 0.98–2.11 |
| Smoking | ||||
| No | 1 | Reference | ||
| In pregnancy | 67 | 21.7 | 2.18* | 1.51–3.15 |
| First trimester | 54 | 17.5 | 2.05* | 1.38–3.05 |
| Second trimester | 50 | 16.2 | 2.14* | 1.41–3.24 |
| Third trimester | 52 | 16.8 | 2.15* | 1.43–3.24 |
| Mother after birth | 89 | 28.6 | 1.66* | 1.21–2.27 |
| Father after birth | 109 | 37.6 | 1.32 | 0.98–1.77 |
| History of asthma | ||||
| Father | 30 | 9.7 | 1.53 | 0.94–2.50 |
| Mother | 33 | 10.6 | 1.57 | 0.98–2.52 |
| Siblings | 27 | 8.8 | 2.17* | 1.25–3.77 |
| History of rhinitis | ||||
| Father | 53 | 17.2 | 1.62* | 1.10–2.37 |
| Mother | 50 | 16 | 1.31 | 0.89–1.92 |
| Siblings | 19 | 6.2 | 3.44* | 1.64–7.18 |
| Pollution | 102 | 32.7 | 1.33 | 0.99–1.79 |
| Mould in home | 63 | 20.3 | 1.21 | 0.85–1.70 |
| Carpet in home | 6 | 1.9 | 0.50 | 0.20–1.24 |
| Pets | ||||
| Cat after birth | 16 | 5.1 | 0.93 | 0.51–1.72 |
| Hamster/rabbit after birth | 6 | 1.9 | 4.18* | 1.03–16.83 |
| Dog after birth | 50 | 16 | 0.76 | 0.53–1.10 |
| Maternal educational level | ||||
| Primary/incomplete secondary (≤11 years) | 1 | Reference | ||
| Complete secondary and higher (≥12 years) | 159 | 50.8 | 0.94 | 0.62–1.42 |
| University | 113 | 36.1 | 0.85 | 0.55–1.31 |
| Paracetamol during pregnancy | ||||
| Never of less than once a month | 1 | Reference | ||
| 1–4 times a month | 52 | 16.8 | 1.32 | 0.90–1.92 |
| Over once a week | 21 | 6.8 | 2.49* | 1.31–4.73 |
Recurrent wheezing (RW) risk factors. Odds ratio (OR) with 95% confidence interval (95%CI).
| n | % | OR | 95%CI | |
|---|---|---|---|---|
| Male | 86 | 62.8 | 1.79* | 1.23–2.60 |
| Exclusive breastfeeding ≤3 months | 82 | 59.9 | 1.53* | 1.06–2.22 |
| Weight at birth | ||||
| 2500–3499g | 1 | Reference | ||
| ≤2499g | 13 | 9.9 | 1.02 | 0.53–1.94 |
| >3500g | 41 | 31.3 | 1.01 | 0.66–1.53 |
| Number of siblings | ||||
| None | 1 | Reference | ||
| 1 | 71 | 51.8 | 0.43 | 0.29–0.64 |
| 2 | 12 | 8.8 | 0.50 | 0.24–1.03 |
| Attending nursery school | 48 | 35.3 | 2.92* | 1.96–4.35 |
| First cold ≤ 3 months | 68 | 49.6 | 2.11* | 1.46–3.04 |
| Eczema in infant | 29 | 21.5 | 1.92* | 1.21–3.04 |
| Smoking | ||||
| No | 1 | Reference | ||
| In pregnancy | 28 | 20.9 | 1.53* | 1.05–2.22 |
| First trimester | 26 | 19.4 | 1.80* | 1.21–2.68 |
| Second trimester | 22 | 16.4 | 1.65* | 1.07–2.55 |
| Third trimester | 21 | 15.7 | 1.48 | 0.95–2.31 |
| Mother after birth | 31 | 22.8 | 1.02 | 0.73–1.42 |
| Father after birth | 47 | 35.9 | 1.09 | 0.85–1.40 |
| History of asthma | ||||
| Father | 18 | 13.2 | 2.15* | 1.21–3.79 |
| Mother | 17 | 12.5 | 1.77* | 1.00–3.14 |
| Siblings | 15 | 11.2 | 2.47* | 1.32–4.63 |
| History of rhinitis | ||||
| Father | 20 | 14.8 | 1.16 | 0.69–1.95 |
| Mother | 22 | 16.1 | 1.23 | 0.75–2.03 |
| Siblings | 6 | 4.5 | 1.46 | 0.59–3.64 |
| Pollution | 45 | 32.8 | 1.17 | 0.90–1.53 |
| Mould in home | 32 | 23.5 | 1.34 | 0.96–1.88 |
| Carpet in home | 4 | 2.9 | 0.92 | 0.32–2.59 |
| Pets | ||||
| Cat after birth | 5 | 3.6 | 0.65 | 0.26–1.61 |
| Hamster/rabbit after birth | 5 | 3.6 | 7.49* | 2.03–27.54 |
| Dog after birth | 24 | 17.5 | 0.93 | 0.63–1.38 |
| Maternal educational level | ||||
| Primary/incomplete secondary (≤11 years) | 1 | Reference | ||
| Complete secondary and higher (≥12 years) | 69 | 50.4 | 0.74 | 0.39–1.39 |
| University | 53 | 38.7 | 0.60 | 0.31–1.15 |
| Paracetamol during pregnancy | ||||
| Never of less than once a month | 1 | Reference | ||
| 1–4 times a month | 26 | 19.3 | 1.91 | 0.88–4.15 |
| Over once a week | 9 | 6.7 | 1.27 | 0.53–2.99 |
No differences were found in the prevalence of wheezing, RW or SW in relation to newborn infant weight, although a correlation to gender was noted. In effect, both wheezing and RW were more frequent in males (OR 1.38, 95%CI [1.05–1.81] and OR 1.79 [1.23–2.60], respectively).
A total of 50.7% of the infants (n=486) received exclusive breastfeeding (EBF) ≤3 months. The incidence of wheezing in these infants was 36.8% (n=179), versus only 28.4% among those who received EBF >3 months (OR 1.47 [1.12–1.93]). In addition, the infants with EBF ≤3 months suffered RW in 16.9% of the cases, versus 11.7% of those who received EBF >3 months (OR 1.53 [1.06–2.22]).
A significant association was observed between infants with a first cold episode in the first three months of life and wheezing (p=0.001)(OR 2.07 [1.56–2.74]) and RW (p=0.001) (OR 2.11 [1.46–3.04]).
A total of 14.5% of the mothers (n=139) smoked during pregnancy. Overall, a very significant association was observed between wheezing in the infant and smoking (OR 2.18 [1.51–3.15]). Significance was likewise observed on independently evaluating the above association during the first, second and third trimesters of pregnancy, or smoking on the part of the mother after birth. Both RW and SW were also very significantly correlated to smoking during pregnancy (OR 1.53 [1.05–2.22] and OR 1.77 [1.20–2.63], respectively). In the third trimester of pregnancy we observed no significant association between RW and maternal smoking (p=0.08) or between maternal smoking after birth and RW or SW (p=0.91 and p=0.23, respectively).
On the other hand, 8.1%, 7.5% and 5.6% of the mothers, fathers and siblings, respectively, had been diagnosed with asthma – a significant correlation being observed between asthma in siblings and wheezing (OR 2.17 [1.25–3.77]), RW (OR 2.47 [1.32–4.63]) and SW (OR 2.48 [1.23–5.01]), as well as between asthma in the mother (OR 1.77 [1.00–3.14]) and father (OR 2.15 [1.21–3.79]) and RW in the first year of life. Likewise, a significant association was recorded between wheezing and a history of allergic rhinitis in the father (OR 1.62 [1.10–2.37]) and siblings (OR 3.44 [1.64–7.18]), as well as between SW and a history of allergic rhinitis in siblings (2.86 [1.19–6.87]).
The association between eczema in the infant and wheezing proved significant in the case of RW and SW, but not for overall wheezing. In this respect, recurrent wheezing was observed in 22.3% of the infants who had suffered eczema, and in 13% of those without skin problems (p=0.005) (OR 1.92 [1.21–3.04]), while SW was recorded in 17.1% of the infants with eczema as opposed to in 8.7% of those without eczema (p=0.003) (OR 2.15 [1.27–3.61]).
A total of 18.4% of the infants attended nursery school. Of these, 49.4% suffered wheezing, versus 28.9% of those who did not attend nursery school (p=0.001) (OR 2.40 [1.71–3.35]). Furthermore, 27.3% of the infants who attended nursery school suffered RW, versus 11.4% of those who did not attend nursery school – the differences between both groups being statistically significant (p=0.0001) (OR 2.92 [1.96–4.35]).
We found no significant association between wheezing and the presence of pets in the home at the time of birth, although a significant correlation was found with the presence of hamsters or rabbits (p=0.03) after birth of the infant in relation to both overall wheezing and to RW.
As regards the relationship between mould in the home and wheezing in the first year of life, we only observed a significant association to SW (p=0.006) (OR 1.68 [1.18–2.38]), but not to overall wheezing or RW.
On examining the relationship between wheezing and the existence of perinatal problems, we identified a significant association between overall wheezing and perinatal factors such as gestational hypertension (OR 1.59 [1.03–2.46]) and foetal suffering or hypoxia (OR 2.05 [1.02–4.12]), as well as between SW and placental problems (OR 3.36 [1.74–6.49]).
On the other hand, in relation to the association between wheezing and paracetamol use, a significant correlation was only observed with overall wheezing and SW (OR 2.49 [1.31–4.73] and OR 4.24 [2.01–8.91], respectively), but not with RW.
Lastly, no significant association was found between overall wheezing and Caesarean section, contraceptive use before pregnancy, or the consumption of “fast food” or the Mediterranean diet during pregnancy.
DiscussionWheezing is one of the most common clinical symptoms in the first year of life. According to the first data of the EISL, a total of 45.2% of all infants experience some respiratory episode characterised by wheezing in the first year of life, and 20.3% suffer three or more such episodes (RW).13 The number of prevalences of wheezing reported within the mentioned study varies greatly from 63.6% in Porto Alegre in Brazil to 12.5% in the city of Mérida in Mexico – the mean prevalence being 47.3% in Latin America versus 34.4% in Europe. Regarding the Spanish centres included in the study, the prevalences of wheezing range from 39.1% and 28.7%, and those of RW between 16.2% and 12.1%, respectively. The data available in our country are similar to those published over a decade ago, when 32.8% of all children were found to have experienced at least one episode of wheezing before 12 months of age. It therefore seems that the prevalence of wheezing has stabilised over the last few years.15
Although the magnitude of the problem in Europe is different from that found in Latin America, the data published by other European studies confirm such observations. This is the case for example of the ALSPAC study,16 conducted in the city of Bristol, where 21.5% of the infants under six months of age were found to have suffered wheezing at least once. Likewise, another study carried out in Belgium reported a 23.5% prevalence of wheezing in the first year of life.17
The data of our study indicate that the prevalences of wheezing and RW in Cantabria (32.7% and 14.3%, respectively), a coastal community in the north of Spain, are similar to those previously reported in different Spanish cities such as Valencia (28.7% and 12.1%) or La Coruña (34.8% and 13.8%), but clearly lower than those registered in Cartagena (39.1% and 16.2%) or Bilbao (38.9% and 18.6%) – all these being coastal cities.13 In relation to non-coastal locations in Spain, our figures are similar to those reported for the province of Salamanca (32.3% and 11.9%),18 and higher than those recorded in the city Alzira in the province of Valencia (25.2% and 11.6%) (although the SR data in this case are similar).19 However, this study only documented the first six months of life, and moreover excluded premature infants. Considering all the data obtained to date, it can be affirmed that no differences have been found in the prevalence of wheezing and RW according to whether the setting is costal or inland, in this particular age population. This situation is in clear contrast with the published prevalences of bronchial asthma in Spain, with very high figures in the coastal regions of Cantabria compared with other inland areas of the rest of the country.20
In coincidence with the already published data of the EISL,13,21 we found the occurrence of a first cold episode in the first three months of life to be a risk factor for wheezing, RW and SW. Most of the wheezing episodes in small children are caused by viruses,5,22 and viral infections probably explain the results referred to children attending nursery school, where coinciding with other studies involving this same age interval,23 we recorded a statistically significant correlation to wheezing, RW and SW. Indeed, the associated risk in this case was found to increase up to threefold in the case of recurrent wheezing.
A total of 42.2% of the infants with wheezing (n=132) were taken to the emergency room (representing 13.7% of the total sample), and 11.5% of the infants with wheezing (n=36) (3.7% of the sample) were admitted to hospital. The fact that four out of every 10 infants with wheezing were taken to the emergency room may be related to the age of the patients and to the great family concern caused by wheezing in the nursing infant, as well as easy accessibility to the public health system in Cantabria, with a very complete Primary Care network and nearby reference hospitals. Nevertheless, the data are similar to those of most Spanish centres in the EISL, where between 41% and 49.5% of the patients were seen to receive emergency care. The only contrasting observation corresponds to Valencia, where a reported 74.4% of all infants with wheezing were taken to the emergency room. Regarding the infants admitted to hospital, the findings range from 7.2% in Bilbao to 14.2% in La Coruña. Our own data fall within the middle range of the figures reported by the published studies.13
Regarding other examined factors which could be related to the presence of wheezing in the first year of life, we found wheezing and RW to be significantly more frequent in males – in coincidence with the observations of other authors.19,21,24 However, we found no association between wheezing and infant weight at birth, in contrast to other investigators who found a low birth weight to imply an increased risk of respiratory infections and wheezing.25 On the other hand, the statistically significant association observed in our study between RW and SW and the presence of eczema in the infant coincides with the data published elsewhere.3,13,24
Regarding breastfeeding, our data coincide with those of other studies13,19,21 in that a statistically significant association was observed between wheezing, RW and SW and exclusive breast feeding (EBF) during ≤3 months. In this respect, it is still a matter of debate whether the prolongation of EBF reduces the frequency of wheezing by constituting a protective factor against infections. A lesser risk of allergic sensitisation has been reported in breastfed infants,26 although other similar recent studies27 have concluded that although breastfeeding affords protection against infections, it could also increase allergies and asthma at later ages.
The association identified between RW and antecedents of maternal asthma, paternal asthma and asthma in the siblings is consistent with the observations of other authors,13,21,28 and some studies moreover point to asthma among the parents (fundamentally maternal asthma) as a major risk factor for atopic wheezing risk in nursing infants.29
In line with the extensive literature relating maternal smoking during pregnancy and tobacco smoke exposure to the development of wheezing in children,6,9,13,30 our study confirms the association between smoking and both overall wheezing and to RW and SW considered individually. On analysing the data according to smoking in each trimester of pregnancy, we have been able to confirm this correlation in all cases except between maternal smoking in the last trimester of pregnancy and RW – probably because of the relatively small number of mothers who smoke, and the fact that no objective method for the detection of smoking was used. On the other hand, maternal smoking after delivery was also significantly associated to the presence of wheezing. All these data reflected by most published studies contribute further support to the new legal regulations designed to reduce smoking, introduced in recent years, and which undoubtedly will modify the prevalence of wheezing and its association to smoking.31
As with other authors,18,19,24 we found no statistically significant association to the presence of pets in the home at or after birth. Nevertheless, on individualising the type of pet, a statistically significant correlation was found between wheezing and RW and the presence of hamsters or rabbits after birth. Although this has also been observed in other studies,18 a larger sample is needed in order to confirm its clinical relevance.
No correlation to university education on the part of the mother was observed,18,19 in contrast to the findings of studies carried out in Latin America.21
Regarding environmental factors, an association was recorded between mould in the home and SW, but not overall wheezing or RW. Different European studies24 and investigations made in Latin America where there are more homes with mould problems,21 have also observed this association.
One of the examined factors was the relationship between paracetamol use and wheezing. This drug is widely used in paediatric patients for the symptomatic treatment of fever and pain, and has been related by many studies to an increased prevalence of asthma, rhinitis and eczema.32 Likewise, other studies have described a correlation between prenatal exposure to paracetamol and the subsequent development of wheezing or asthma,11,33 although it has also been reported that this association may be dose-dependent34 and may even disappear on taking into account possible confounding factors such as the frequency of infectious processes and their increased expressivity in asthmatic children.35 Although our data indicate that paracetamol use more than once a week during pregnancy is significantly associated to the prevalence of overall wheezing and SW (but not RW), the descriptive observational nature of our study does not allow us to demonstrate a cause–effect relationship. Recently, the Spanish Association of Pediatrics has reviewed this topic and considers that the existing information is not solid enough to advise against paracetamol use during pregnancy or in infants with asthma or at risk of developing asthma.36
One of the possible limitations of this study refers to the validity of the information supplied by the parents through the EISL questionnaire, which nevertheless has been correctly validated.14 Another limitation which could alter the results is the seasonal and annual variation and the virulence of the viral processes in the origin of wheezing. This has been largely compensated by the fact that we included infants born in different seasons and years. On the other hand, the main strong points of the study include the fact that it was carried out in the Primary Care setting, which is the first healthcare level for the paediatric population; the participation of both rural and urban Primary Care Centres; and the use of a sample size large enough to analyse the different risk factors investigated.
In conclusion, in the Community of Cantabria, one third of the studied children suffered wheezing during the first year of life, with a significant impact upon patient feeding, sleep and quality of life, and also upon the family. Wheezing was significantly more frequent among males, infants exclusively breastfed for less than three months, infants with a first cold before three months of age, infants attending nursery school, with eczema or a family history of asthma or allergic rhinitis, and infants with mothers who had smoked during pregnancy or after delivery. In 14.3% of the cases wheezing was seen to be recurrent.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
FundingThis project has been financed by the Fundación Ernesto Sánchez-Villares, Grant-Research aid 2011 corresponding to the XIV Call for Aids.
Conflict of interestThe authors have no conflict of interest to declare.
The authors thank all the paediatricians of the Primary Care network of Cantabria who have collaborated in the data collection process, for their effort, commitment and generosity.
Estudio Internacional de Sibilancias en Lactantes (International Study of Wheezing in Infants). Primary Care Centres in Cantabria (Spain).