β2-adrenoreceptor (β2-AR) agonists and glucocorticoids (GCS) were shown to induce IgE synthesis in human PBMCs. Serum total IgE levels are associated with single nucleotide polymorphisms (SNPs) of the β2-AR gene. We aimed to assess the association of the effect of fenoterol (β2-AR agonist) on IL-4-driven and budesonide-induced IgE synthesis with genetic variants of β2-AR.
MethodsThe study included 25 individuals: 13 with allergic asthma and/or allergic rhinitis and 12 healthy volunteers. PBMCs were cultured with IL-4, fenoterol and/or budesonide, and IgE concentrations in supernatants were assessed. Five SNPs in positions: −47, −20, 46, 79 and 252 of β2-AR were determined by direct DNA sequencing.
ResultsIn −47 T/T and −20 T/T patients, incubation with fenoterol resulted in decreased IgE production, whereas in −47 C/T and −47 C/C as well as in −20 C/T and −20 C/C individuals, it was enhanced. In contrast to fenoterol, budesonide-induced IgE synthesis was significantly increased in −47 T/T and −20 T/T patients as compared to −47 C/T, −47 C/C, −20 C/T and −47 C/C individuals. Polymorphisms in positions 46, 79 and 252 were not associated with fenoterol- or budesonide-modulated IgE synthesis. No differences in the distribution of IgE synthesis was seen between atopic and non-atopic individuals carrying the same alleles.
ConclusionsThe differential effect of β2-agonists and GCS on IgE synthesis may be associated with genetic variants of promoter region of the β2-AR gene.
Immunoglobulin E (IgE) is a key molecule engaged in allergic responses. In vitro studies show that β2-adrenoreceptor (β2-AR) agonists and glucocorticoids (GCS), which are the most effective treatment for asthma, induce IgE synthesis by human peripheral blood mononuclear cells (PBMCs) and B cells.1,2 Our study demonstrated that inhaled GCS, budesonide, induces IgE synthesis by PBMCs in both allergic patients and healthy subjects.3 Tantisira et al. confirmed that inhaled GCS leads to an increase in serum total IgE concentrations in children, depending on variants of the polymorphic Fc¿RII receptor.4 We also found that serum total IgE concentrations in patients with grass pollen allergy may be associated with polymorphic β2-AR.5 Similarly, Dewar et al. found a significant linkage between total IgE and 27 Gln receptor variant.6 A positive association between the presence of a haplotype containing 27Gln and increased levels of IgE was also found in CAMP children.7
In the β2-AR gene, more than 10 single nucleotide polymorphisms (SNPs) have been identified.8,9 SNPs at nucleotides 46 and 79 lead to changes in amino acid residues at positions 16 (16 Arg/Gly), 27 (27 Gln/Glu) and 164 Tre/Ile. SNPs in the -47 and -20 positions, being in a strong linkage disequilibrium, are located in the 5′ leader region of β2-AR,10 which has a short open reading frame, and encodes a 19-amino acid peptide termed 5′ leader cistron (5′LC). This region regulates mRNA translation and controls the cellular expression of β2-AR.11 The mutation at position 19 causes a change from Cys to Arg.12 An in vitro study indicates that expression of the 5′ LC Cys19 allele in airway smooth muscle was approximately two-fold greater than that of the 5′ LC Arg19 allele.12 The −47 C/C variant, which encodes 5′LC with Arg19, increases its ability to exert translational inhibition of β2-AR expression.11,13 All SNPs are considered to be involved in the regulation of β2-RA surface expression: B2-AR SNPs in positions −47, −20, 46, 79, 252 might be associated with an asthmatic phenotype and bronchial hyperresponsiveness.14–17
Our previous study demonstrated that in allergic individuals, total serum IgE levels were associated with β2-AR polymorphism.5 The aim of the present study is to analyse the association of in vitro β2-AR agonist- and glucocorticoid-induced changes in IgE production by PBMCs with β2-AR polymorphisms in positions −47 and −20 of the promoter region and 46, 79 and 252 of the protein coding region.
Materials and methodsPatientsThe study included 25 subjects: 13 adult patients with bronchial asthma and/or allergic rhinitis and positive skin prick tests (SPT) to relevant allergens, and a group of 12 healthy volunteers, with no history of allergy and with negative SPT to a panel of inhaled allergens (Table 1). Patients received neither inhaled glucocorticoids nor long-acting β2-agonists for 48 hours, as well as anti-histamines for seven days prior to the study. Written consent was obtained from the subjects before participation in the study. The study was approved by the Local Ethics Committee of the Medical University of Lodz, Poland.
Clinical characterization of patients included to the study (A). Genotypic frequencies of β2-AR loci −47, −20, 46, 79 and 252 in groups of studied patients (B); n=25.
| (A) | |||
| Allergic patients | Healthy subjects | p | |
| Number (n) | 13 | 12 | >0.05 |
| Sex | |||
| Women | 5 | 9 | >0.05 |
| Men | 8 | 3 | >0.05 |
| Age (yrs) | |||
| Mean (range) | 32.8±2.9 (23–58) | 31.4±2.7 (23–57) | >0.05 |
| Serum t IgE (kU/L) | |||
| Mean range | 2765±124.3 (25–1690) | 30.6±10.6 (2–123) | <0.01 |
| (B) | ||||
| Position in gene | Position in protein | Allel | No | % |
| −47 | 19 Cys/Arg | T/T | 15 | 60 |
| C/T | 6 | 24 | ||
| C/C | 4 | 16 | ||
| −20 | C/C | 7 | 28 | |
| C/T | 9 | 36 | ||
| T/T | 9 | 36 | ||
| 46 | 16 Arg/Gly | A/A | 4 | 16 |
| A/G | 10 | 40 | ||
| G/G | 11 | 44 | ||
| 79 | 27 Gln/Glu | C/C | 11 | 44 |
| C/G | 7 | 28 | ||
| G/G | 7 | 28 | ||
| 252 | 164 Thr/Ile | G/G | 16 | 64 |
| A/G | 8 | 32 | ||
| A/A | 1 | 4 | ||
Blood collected in Sodium-Heparin vacuum tubes by venous puncture was diluted in phosphate-buffered saline (1:3) (PBS) (Biomed, Lublin, Poland) and centrifuged on Histopaque 1077 density gradient (Sigma–Aldrich, St. Louis, MO, USA) at 800g. Interphase cells were washed three times in PBS and re-suspended in IMDM medium (Iscove's Modified Dulbecco's Medium) consisting of 2mM l-glutamine, penicillin (100U/ml), streptomycin (100μg/ml) (Sigma–Aldrich, St. Louis, MO, USA) and 10% foetal bovine serum (FBS). The cell viability as assessed by trypan blue exclusion method always exceeded 95%. Cells (2×10.6cells/ml) were then incubated in tubes (Sarsted, Nümbrecht, Germany) at 37°C in 5% CO2 and 95% humidity with either human recombinant IL-4 (10ng/ml), budesonide (10nM) (Sigma–Aldrich, St. Louis, MO, USA) or fenoterol (10nM) (Sigma–Aldrich, St. Louis, MO, USA) for 11 days. After 11 days of culture, the cell-free supernatants were collected and frozen at −70°C in order to assess IgE concentration.
IgE measurementTotal IgE concentrations in the supernatants from PBMC cultures and in the sera of patients were determined using the UniCAP fluoroimmunoenzymatic method (Pharmacia, Upsala, Sweden) according to the manufacturer's protocol.
The results of IgE synthesis in groups of patients with different genotypes are presented as values calculated by the subtraction of agent-induced IgE synthesis values from the control IgE synthesis values [delta IgE (kU/L)].
β2-Adrenoceptor genotypingGenomic DNA was extracted from peripheral blood using “Easy Blood DNA prep” (A&A, Gdansk, Poland) according to the original protocol. The presence of β2-AR single nucleotide polymorphisms in positions −47, −20, 46, 79 and 252 was determined by direct sequencing of PCR products obtained using the following primers: 5′-CTG AAT GAG GCT TCC AGG CGT-3′ and 5′-ACA ATC CAC ACC ATC AGA AT-3′. The 584-bp PCR products were purified with a commercial kit (Qiagen, Valencia, CA, USA) and sequenced using a fluorescently labelled dye terminators technique (“Big-Dye Terminator Cycle Sequencing”, Applied Biosystems, Foster City, CA, USA) in an API Prosm 310 capillary sequencer (Applied Biosystems). Ambiguous samples were sequenced in both directions.
Statistical analysisThe results are presented as mean±SD for variables with a normal distribution of values, or as a median of quartile range for the remaining cases. The distribution of particular variables was verified by the Shapiro–Wilk test. If the results demonstrated normal distribution and homogenous variance, the significance of the differences between the two groups was estimated using the Student t-test for independent trials or ANOVA with post hoc Tukey test. However, if any of these criteria was not fulfilled, a Wilcoxon test for independent trials within the group or Mann–Whitney-U test for analysis of differences between two groups were used, as well as a Kruskal–Wallis with post hoc Connover–Inman test for variance analysis. All statistical evaluations were performed using Statistica software (StatSoft, Inc., Tulusa, OK, USA).
ResultsThe effect of fenoterol and budesonide on IgE synthesis by PBMCsIncubation of PBMCs with IL-4 resulted in a significant increase in mean generation of IgE by PBMCs (from 0.2±0.28 to 0.58±0.5kU/L, p<0.01, n=25) (Table 2). No difference in mean IgE synthesis was seen in response to the addition of IL-4 alone or IL-4 plus fenoterol. However, individual data showed both an increase and decrease of IgE production. Budesonide further stimulated IL-4-induced IgE synthesis by PBMCs from 0.58±0.28 to 8.85±9.84kU/L (p<0.001, n=25). Mean IgE synthesis in response to fenoterol added to budesonide and IL-4 was similar to budesonide alone. Individual data showed both strong increases and decreases of IgE production. Neither fenoterol nor budesonide alone or together affected IgE synthesis by PBMCs without the presence of IL-4.
The effect of fenoterol and budesonide on IL-4-induced IgE synthesis by PBMCs in vitro. (*p<0.01 for IgE synthesis induced by IL-4 compared to media-controlled; #p<0.001 for IgE synthesis induced by budesonide and IL-4 compared to IL-4 alone), n=25.
| Condition | Mean±SD (kU/L) | Median | Range (min–max) |
| Media-controlled | 0.28±0.28 | 0.0 | 0.0–0.8 |
| IL-4 (10ng/ml) | 0.58±0.50* | 0.5 | 0.0–1.8 |
| IL-4 (10ng/ml)+Fen (10nM) | 0.58±0.51 | 0.6 | 0.0–1.9 |
| IL-4 (10ng/ml)+Bud (10nM) | 8.85±9.84# | 4.4 | 0.0–31.0 |
| IL-4 (10ng/ml)+Fen (10nM)+Bud (10nM) | 11.88±18.29 | 4.2 | 0.0–75.0 |
As in vitro experiments have indicated that individual variations in IgE synthesis have been found to occur in response to fenoterol administration, this study addressed whether β2-AR genetic polymorphisms may be associated with these differences. The β2-AR genotype frequencies for all tested polymorphic loci are shown in Table 1B.
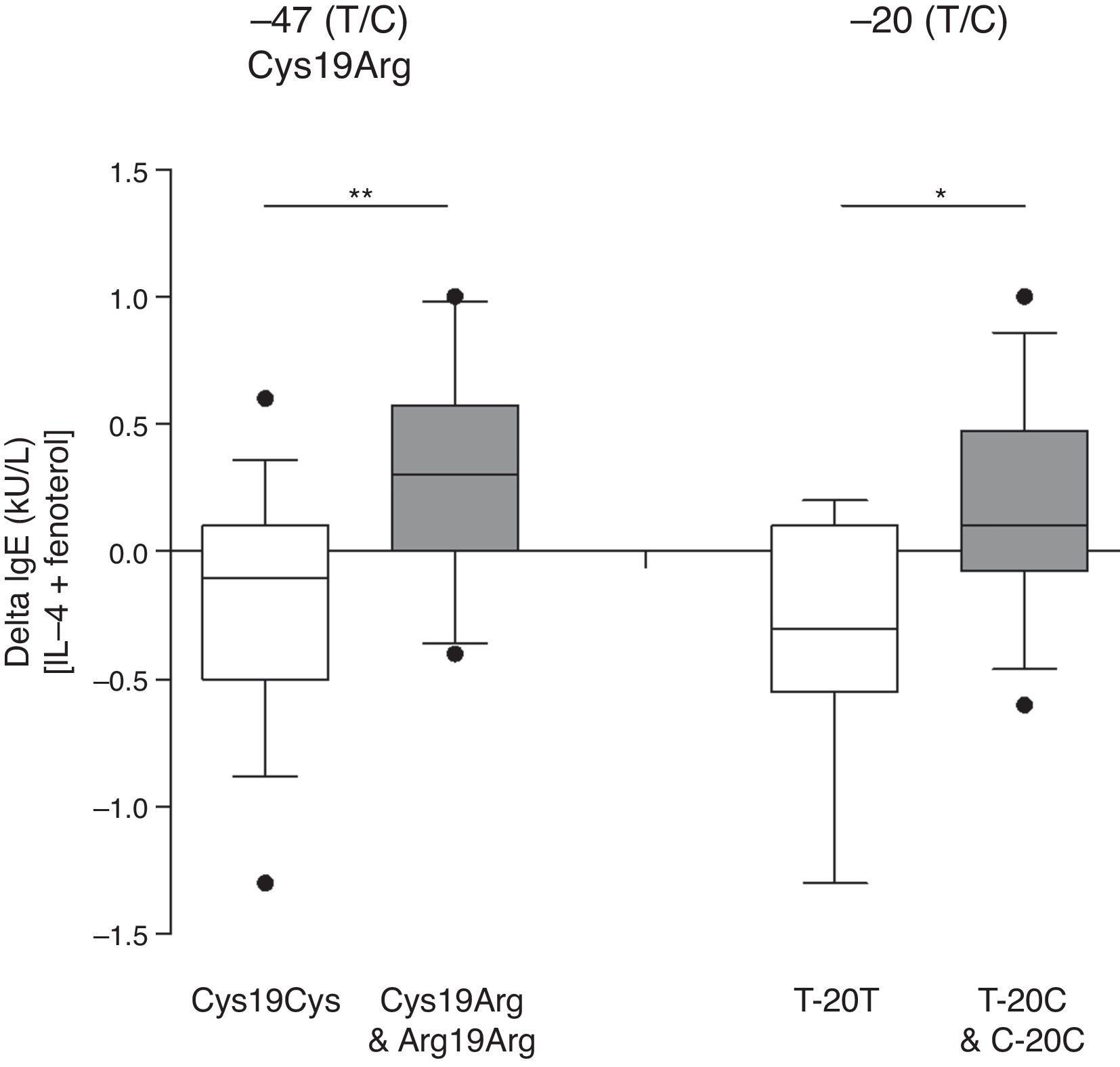
While the mean IgE synthesis upon incubation with fenoterol (10nM) was decreased in patients with homozygotic genotype −47 T/T (Cys19Cys), it was enhanced in those with −47 C/T (Cys19Arg) and −47 C/C (Arg19Arg) taken together (p=0.01) (Fig. 1). A similar association was observed with regards to −20 T/C SNP: in patients with homozygotic genotype −20 T/T IgE, synthesis in the presence of fenoterol (10nM) was decreased, whereas it was enhanced in −20 C/T and −20 C/C individuals taken together (p<0.05) (Fig. 1).
The effect of β2-AR polymorphisms in positions −47, −20 on fenoterol-induced changes in IgE synthesis by PBMCs in vitro. The graph depicts values calculated by the subtraction of fenoterol-induced IgE synthesis values from IL-4-induced IgE synthesis values. Data are represented in a box and whiskers plot showing median and 10–90 percentiles. The outliers are shown as single points beyond the whiskers (*p<0.05, **p=0.01).
None of the polymorphic variants in positions 46, 79 and 252 significantly affected the influence of fenoterol on IgE synthesis (Fig. 4A). Additionally, none of the different genotypes in positions −47, −20, 46, 79 and 252 significantly affected media-controlled or IL-4-induced IgE synthesis by PBMCs (data not shown).
The influence of β2-AR genetic polymorphisms in the 5′ LC promoter region in moderating the effect of budesonide on PBMC IgE synthesisBudesonide exposure was seen to increase IgE synthesis in subjects with the homozygotic genotype −47 T/T (Cys19Cys) as compared to those with −47 C/T (Cys19Arg) and −47 C/C (Arg19Arg) taken together (p<0.05) (Fig. 2). Similarly, the increase of IgE synthesis in the presence of budesonide (10nM) was lower in patients with homozygotic genotype −20 T/T, than in those with −20 C/T and −20 C/C taken together (p<0.05) (Fig. 2). Variants of both polymorphic β2-AR genes in nucleotides 46, 79 and 252 did not significantly moderate the influence of budesonide on IgE synthesis (Fig. 4B).
The effect of β2-AR polymorphisms in positions −47, −20 on budesonide-induced changes in IgE synthesis by PBMCs. The graph depicts values calculated by the subtraction of budesonide-induced IgE synthesis values from IL-4-induced IgE synthesis values. The results are represented in a box and whiskers plot showing median and 10–90 percentiles. The outliers are shown as single points beyond the whiskers. (*p<0.05).
The results of the study show that fenoterol both increased and decreased budesonide-induced IgE synthesis by PBMCs in individuals. However, no differences in IgE synthesis were observed between subjects carrying homozygotic genotype −47 T/T (Cys19Cys) and those carrying −47 C/T (Cys19Agr) and −47 C/C (Arg19Arg) taken together (p>0.05), −20 T/T, and −20 C/T and −20 C/C taken together (Fig. 3) as well as in individuals carrying different variants of SPNs: 46, 79 and 252 (Fig. 4C).
The effect of β2-AR polymorphisms in positions −47, −20 on fenoterol–budesonide-induced changes in IgE synthesis by PBMCs. The graph depicts values calculated by the subtraction of fenoterol–budesonide-induced IgE synthesis values from budesonide-induced IgE synthesis values. The results are represented in a box and whiskers plot showing median and 10–90 percentiles. The outliers are shown as single points beyond the whiskers. (ns, p>0.05).
The relation between β2-AR polymorphisms in positions 46, 79 and 252 and fenoterol- (A) budesonide- (B) and fenoterol–budesonide-induced (C) changes in IgE synthesis by PBMCs. The graph depicts values calculated by the subtraction of fenoterol–budesonide-induced IgE synthesis values from budesonide-induced IgE synthesis values. The results are represented in a box and whiskers plot showing median and 10–90 percentiles. The outliers are shown as single points beyond the whiskers (values of all compared groups are statistically insignificant, p>0.05).
No differences were found in response to both fenoterol and budesonide between atopic and non-atopic individuals carrying the same alleles (data not shown).
The influence of β2-AR genetic polymorphism in the 5′ LC promoter region on serum total IgE concentrationIn atopic patients, serum total IgE concentrations were significantly higher than in healthy subjects (Table 1A). No significant differences were seen in mean serum total IgE levels between patients with the homozygotic genotype −47 T/T and individuals carrying −47 C/T (Cyc19Cys versus Cys19Arg and Arg19Arg groups [IgE level median {kU/L}; 10% percentile and 90% percentile]: 54; 4.65–421.5 versus 28; 2.4–291.0, respectively; p>0.05) and −47 C/C (T-20T versus T-20C and C-20C groups [IgE level median {kU/L}; 10% percentile and 90% percentile]: 52; 4.3–395.0 versus 28; 4.1–342.0; respectively; p>0.05). Similarly, serum total IgE concentrations in groups of individuals with analysed SNPs in position 46 (Arg16Arg and Arg16Gly versus Gly16Gly groups [IgE level median {kU/L}; 10% percentile and 90% percentile]: 45.5; 5.1–346.0 versus 32.5; 2.3–426.9; respectively; p>0.05), in position 79 (Gln27Gln versus Gln27Glu and Glu27Glu groups [IgE level median {kU/L}; 10% percentile and 90% percentile]: 56.0; 9.4–437.4 versus 25.0; 9.2–181.4; respectively; p>0.05) and in position 252 (Tre164Tre versus Tre164Ile and Ile164Ile groups [IgE level median {kU/L}; 10% percentile and 90% percentile]: 56.0; 4.4–336.2 versus 32.0; 4.3–448.0; respectively; p>0.05) were also comparable.
DiscussionOur study demonstrates that fenoterol, a β2-adrenoreceptor agonist, and budesonide may influence IgE synthesis by PBMCs in vitro. However, both increases and decreases in IgE synthesis may be observed in individual subjects. Furthermore, fenoterol was seen to affect budesonide-induced IgE synthesis. To the best of our knowledge, this is the first evidence of interaction between the β2-adrenoreceptor agonist and glucocorticoid with regards to IgE synthesis in vitro.
Coqueret et al. demonstrated that salbutamol and fenoterol increased IgE synthesis by PBMCs and B cells, and suggested that this enhancement was related to cAMP release.18 It has been previously demonstrated by the present authors that budesonide, an inhaled glucocorticosteroid, strongly induces IgE synthesis by PBMCs.3 Increased IgE synthesis has also been observed upon systemic exposure to glucocorticoids. However, the synergistic effect of β2-agonist and GCS has not been studied. Our study shows that fenoterol may significantly affect IgE generation induced by budesonide. Individually, fenoterol was found to enhance or decrease budesonide-induced IgE generation. This observation is in line with studies showing both synergistic and antagonistic interaction between β2-AR agonists and glucocorticoids in both clinical19,20 and experimental21–23 settings.
Our previous study identified an association between serum total IgE concentration and β2-AR polymorphisms, suggesting that β2-AR is involved in the regulation of IgE synthesis.5 In the present study, the five most common single nucleotide polymorphisms at nucleotide positions −47, −20, 46, 79 and 252 were analysed. The β2-AR genotype frequencies for all tested polymorphic loci were comparable to the frequencies described previously in the larger group of patients.5
The results of this study demonstrate that the presence of T/T in both the −47 and −20 nucleotides of the β2-AR promoter region tend to decrease IgE synthesis in response to fenoterol. In contrast, the C/T and C/C alleles in nucleotides −47 and −20 seem to determine the increase of IgE generation by fenoterol. No variants in positions 46, 79 and 252 located within the coding part of the β2-AR gene were found to have an impact on the modulation of IgE synthesis by fenoterol. The differential effect of fenoterol on IgE synthesis may depend on the presence, or absence, of particular amino acids within the 5′-leader cistron, which is the product of the β2-AR open reading flame (ORF). Carrying at least one Arg in position 19 of 5′-LC (19 5′LC Cys/Arg and 19 5′LC Arg/Arg) may increase IgE synthesis in response to fenoterol. However, on the contrary, having only Cys (5′LC Cys/Cys) may decrease IgE generation in response to fenoterol. It is important to note that 5′ LC Arg19 or 5′ LC Cys19 variants have already been shown to differentially regulate β2-AR gene expression: The −47 C/C variant, which encodes 5′LC with Arg19, increases its ability to exert translational inhibition of β2-AR expression. It could not be confirmed whether −20 C/T induces changes in the amino acid sequence of 5′ LC. Our results taken as a whole indicate that the β2-AR promoter region coded by 5′ LC may be associated with the effect of fenoterol on IgE synthesis.
It may be assumed that the divergent modulation of IgE synthesis by fenoterol might result not from 5′LC, but also from polymorphisms in the other eight positions of the β2-AR promoter: −367, −406, −468, −654, −709, −1023, 1343 and −1429 encoding AP2 (activating protein-2), p35, NF-IL-6 (nuclear factor-IL-6) and steroid binding sites, all evidenced to be in a strong linkage disequilibrium with polymorphism in −47 and −20.24,25 No difference was seen regarding the influence of β2-AR SNPs on fenoterol-modulated IgE synthesis in atopic and non-atopic individuals.
The results of the analysis concerning the significance of β2-AR SNPs in the induction of IgE synthesis by budesonide were surprising. Budesonide-induced IgE synthesis was found to be significantly higher in −47 T/T and −20 T/T individuals than in C/T and C/C patients. Carrying at least one Arg in position 19 of 5′-LC was associated with weakening the stimulatory effect of budesonide on IgE synthesis. However, having only Cys in position 19 of the 5′-LC region enhanced the stimulatory effect of budesonide on IgE synthesis. No difference was found in the influence of β2-AR SNPs on glucocorticoid-modulated IgE synthesis in atopic and non-atopic individuals.
Certain associations between the response to glucocorticoids and β2-AR polymorphism have been reported previously. Dexamethasone reverses β2-AR desensitisation in HASM cells (human airway smooth muscle cells) without the Arg19 allele in the 5′ leader peptide of β2-AR, whereas in cells containing at least one arginine, this effect was not observed.26 No difference was seen in the influence of β2-AR SNP on budesonide-induced IgE synthesis in atopic and non-atopic individuals. These results indicate that the β2-AR promoter region coding 5′ LC may modulate the effect of budesonide on PBMC IgE synthesis.
As both IgE synthesis modulated by fenoterol and budesonide did not differ in individuals having different variants in positions 46, 79 and 252 of the coding region of β2-AR, these polymorphisms seem not to play any important role in this phenomenon. Additionally, no β2-AR polymorphism in any studied position, −47, −20, 46, 79 or 252, was found to have any impact on budesonide-induced IgE synthesis modulated by fenoterol. However, Moore at al. recently demonstrated that albuterol stimulation, evoked by the exposure of cells to dexamethasone, is greater in adenyl cyclase Met 772 variant cells in comparison to wild type.24 Furthermore, in children receiving budesonide, those with the Met772 genotype were associated with an increased bronchodilator response to albuterol when compared with those with the WT AC9 genotype.27 In our study, no difference was found in the influence of β2-RA SNP on the modulation of budesonide-induced IgE synthesis by fenoterol in atopic and non-atopic individuals. No association was found between SNPs of β2-AR and serum total IgE synthesis.
It is worth asking the question as to whether GCS and β2-AR agonists may affect IgE synthesis in vivo. The effect of systemic GCS and inhalant budesonide on the elevation of total IgE levels in sera of asthma patients has already been shown.2,4 Furthermore, Tantisira et al. observed that increased concentrations of total IgE in sera of asthmatic children taking budesonide were associated with polymorphic variants of Fc¿RII gene, suggesting that IgE production may depend on the polymorphism of certain genes. On the other hand, topical budesonide did not affect nasal IgE levels in hay fever patients.28 As the data are not consistent, it would be worth checking whether inhaled GCS may affect bronchial IgE levels in asthmatic patients. As far as β2-AR agonists are concerned, systemic salbutamol failed to affect mean total serum IgE levels in vivo.29 It is unknown, whether it might have been associated with β2-AR promoter polymorphism. Secondly, it should be pointed out that epidemiological surveys on genetic polymorphisms are usually performed on the larger scale, indeed. However, each group with β2-AR polymorphic variants in our experimental study consisted of 10–15 patients. This number of subjects is generally acceptable for the interpretation of the results from in vitro studies. Furthermore, the β2-AR genotype frequencies for polymorphic loci were comparable to the frequencies described in the larger group of patients.5 Finally, it should be underlined that both GCS and β2-AR agonists are the most effective and commonly used long-term control medications for asthma.
The associations of β2-AR promoter region polymorphisms in positions −47 and −20 with both fenoterol-modulated and budesonide-induced PBMC IgE synthesis confirm the role played by β2-AR activity in IgE synthesis. Furthermore, it may suggest that genetic individual variants of the β2-AR promoter region may determine the response to both β2-AR agonists and GCS in clinical situation in asthmatic patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
The study was supported by the Polish Research Committee grant no. N402071 31/2261 and Medical University of Lodz Research Fund no. 503-1026-1.