INTRODUCTION
Eosinophilic inflammation plays an important role in the pathogenesis of asthma1. Increased number of eosinophils are detectable in bronchial biopsy specimens and in induced sputum from patients with asthma2. Eosinophils produce and release cytotoxic proteins, oxygen metabolites and lipid mediators such as cysteinyl leukotrienes (CysLTs), which are considered to cause prominent tissue damage and increased bronchial hyperresponsiveness in asthmatic patients. Indeed, in vivo and in vitro evidence supports the role of CysLTs as important mediators of asthma; they are able to contract bronchial smooth muscle, enhance mucus secretion, increase microvascular permeability, and stimulate proliferation of fibroblasts, and smooth muscle cells3.
So far the mechanism controlling the resolution of eosinophilic inflammation are poorly understood. Apoptosis, a form of programmed cell death, may be important in limiting tissue injury and determining whether inflammation persists or resolves. Apoptosis facilitates ingestion of intact eosinophils by macrophages, thereby preventing the release of their toxic contents. It is thus likely that eosinophil apoptosis could not only play a key role in the resolution of acute and chronic inflammation but it could also change the natural course of the allergic inflammatory disease4-7.
It has been reported that inhaled corticosteroids induce eosinophil apoptosis and their phagositosis by macrophages9-11. On the other hand CysLT receptor antagonists inhibit the effects of leukotrienes by blocking CysLT receptor-1. Treatment with CysLT receptor antagonists decreases the number of eosinophils in peripheral blood and sputum of asthmatic patients but the exact mechanism of this effect is not fully explained12,13. Induction of apoptosis may be a mechanism for CysLT receptor antagonists in controlling tissue eosinophilia, however to our knowledge their effect on eosinophil apoptosis has not been studied until now.
The aim of this study was to assess the effect of montelukast on eosinophil apoptosis in a group of patients with mild persistent asthma and to compare it with the apoptotic effect of fluticasone propionate.
METHODS
Patient selection
Randomly selected mild persistent asthmatic patients who admitted to our outpatient clinic between January 2001 and January 2002 were included in the study. Asthma diagnosis was based on a history of recurrent symptoms of wheezing, shortness of breath, cough and demonstration of objective signs of reversible airway obstruction by means of at least 12 % increase in FEV1 after 15 minutes with an inhalation of 200 μg salbutamol or a PC20 methacholine < 8mg/ml as stated by the American Thoracic Society and International Asthma Guidelines14. Asthma severity was determined by the frequency of asthma symptoms, pulmonary function tests, and medication requirements according to the international guidelines by a chest physician/allergolog. Selected patients had not been taking inhaled corticosteroids, leukotriene receptor antagonists, theophylline and long acting beta2 agonists within the preceeding 12 months of the study. All subjects had been free of airway infection for at least 6 weeks before investigation. The study protocol was approved by local ethical committee and all participants gave written informed consent.
Study design
Before randomization subjects' characteristics were documented by a questionnaire. Allergy prick tests with common allergen extracts were performed at baseline. Following sputum induction procedure patients were randomly divided into two groups whether to take montelukast 10 mg/day orally (Singulair ®, Merck Sharp&Dohme) or inhaled fluticasone propionate 250 μg/day (Flixotide®, GlaxoSmithKlein) for 4 weeks. Sputum induction and evaluation was made again after this treatment period. At baseline and in the end of 4 weeks spirometry was performed. Serum was collected for soluble Fas ligand assay at baseline and following treatment in both groups besides a control group (n = 8).
Sputum induction
Sputum induction was performed between 9.00 and 10.00 AM according to the method proposed by Pin and coworkers and slightly modified and adapted to Pizzichini and coworkers' method2,15. Before inhalation of hypertonic saline solution, all patients inhaled two puffs of salbutamol (200μg) and 10 minutes later underwent spirometry. Then an aerosol of sterile 3 % saline solution was generated by an ultrasonic nebuliser (particle diameter 0.5-6 μ, 3cc, minimum spray time: 3 dk, oscillation frequency: 1.63 MHz) and inhaled for 7 minutes through a mouthpiece without a valve or nose clip. If the patient could not expectorate at this step the concentration of saline was increased gradually according to the decrease in FEV1 measurements from baseline. In case of < 10 % fall in FEV1 concentration of saline was increased from 3 % to 4 % and then to 5 %. If the FEV1 fell by 10-19 % from the post-bronchodilator value the concentration of saline was not increased. If the FEV1 fell by > 20 % or if troublesome symptoms occured nebulisation was discontinued. After each period of inhalation subjects were asked to rinse their mouth and throat carefully, swallow the water and blow the nose to minimize contamination with saliva and postnasal drip. Then they were instructed to try to cough sputum into a container.
Sputum examination
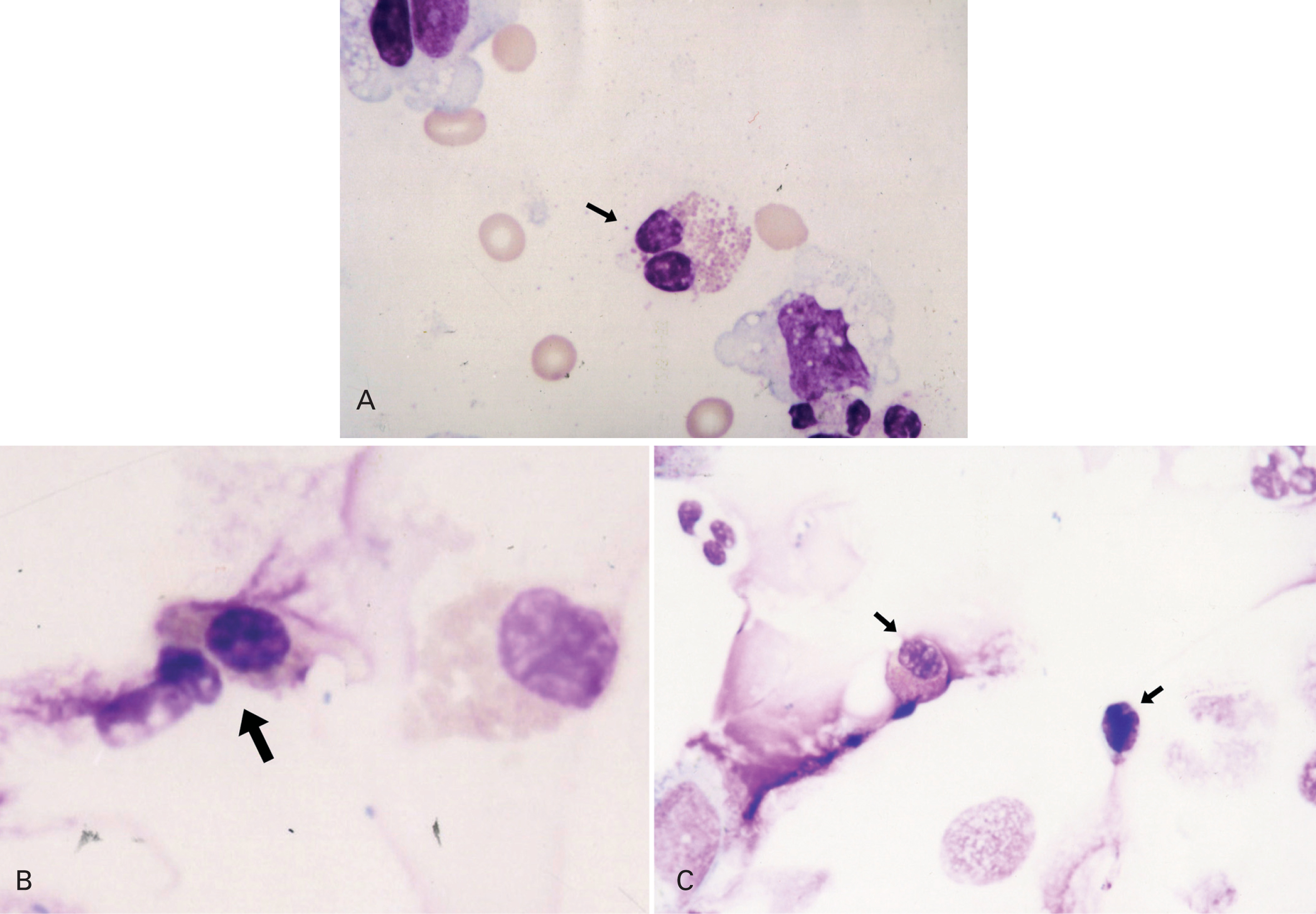
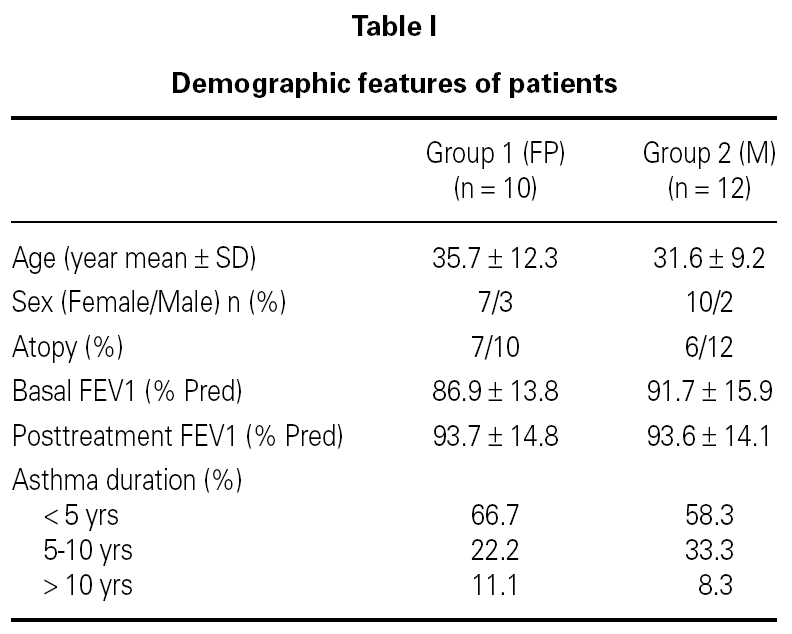
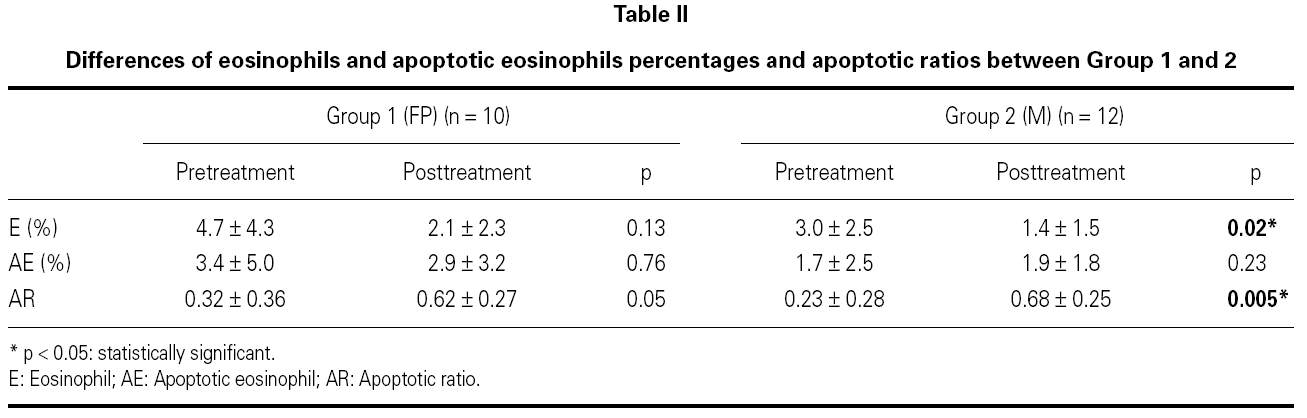
Sputum was processed as soon as possible within 15 minutes according to the method proposed by Pizzichini and coworkers2. It was poored into a Petri dish and all portions that macroscopically appeared free of salivary contamination were placed in a 15 ml polystyrene tube using a straight blunted 115 mm forceps. This selected portion was treated with 4 times the volume of dithiothreitol (DDT) freshly diluted to 0.1 % by the addition of distilled water, to dissociate the disulfide bonds of the mucus. The mixture was vortexed for 15 seconds and gently aspirated in and out of a Pasteur pipette to ensure mixing. To stop the effect of DDT on the cell suspension a further 4 volumes of Dulbecco phosphate-buffered saline (D-PBS) was added. The suspension was filtered through 48 mm nylon gauze to remove cell debris and mucus. The resulting clear suspension was centrifugated at 790 X g for 10 minutes and the supernatant was aspirated and stored in Eppendorf tubes at 70 C for later assay. The pellet was resuspended in a volume of D-PBS, 200-600 μl. Seventy-five microliters of the cell suspension adjusted to 1.0 x 106/ml were placed into cups of Shandon III cytocentrifuge and two coded cytospins were prepared at 450 rpm for 6 minutes. The slides were air-dried and stained by Wright's stain. Morphologic changes of apoptotic eosinophils were assessed by the use of light microscopy. Presence of cell shrinkage and nuclear coalescence ie, shift from bilobed to monolobed nucleus were used to identify apoptotic eosinophils in cytospin preparations from sputum samples. The features of a normal and apoptotic eosinophil in sputum from an asthmatic study patient are shown in figure 1A and 1B. Apoptotic eosinophils were estimated by counting 300 cells in each slide and expressed as the apoptotic ratio [(%apoptotic eosinophils)/(%apoptotic + normal, bilobed eosinophils)]. Slides were coded and cell counts were performed by two expert pathologists who had no knowledge about the clinical characteristics of patients.
Figure 1.--A) Normal eosinophil. B) and C) Apoptotic eosinophils.
Serum soluble Fas ligand assay
Serum soluble Fas ligand concentrations were measured by the use of Enzyme-linked immunosorbent assay (ELISA) (Bender MedSystems, humansFas ligand BMS260/2, Vienna, Austria). Soluble Fas ligand levels of the patients were compared with healthy controls.
Statistical analysis
Data were analysed using the SPSS-PC package, Windows 7.5 version. Results are presented as mean ± SD. Mann-Whitney U test used to study differences between the two independent groups. Comparisons between data from two dependent groups were analysed using Wilcoxon signed-rank test. Values of p < 0.05 were considered statistically significant.
RESULTS
Study groups
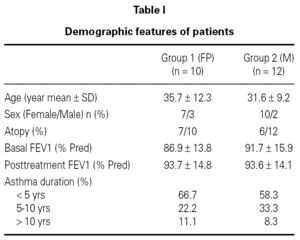
A total of 24 patients were included in the study. Following the baseline sputum induction procedure 2 patients were excluded as they couldn't succesfully produce adequate samples. Remaining 22 patients were randomly divided into two groups: Group 1 (n = 10) was treated with fluticasone dipropionate and Group 2 (n = 12) was treated with montelukast. Demographic characteristics of the study groups are shown in table I.
Safety and success of sputum induction
Sputum induction was successfully performed in the study groups without any complication including bronchoconstruction. Nineteen subjects produced adequate samples at the first attempt. The rest with inadequate samples at the first occasion produced better quality sputum at the following second attempt.
Comparison of two groups by means of asthma history and FEV1 values
There was no difference in FEV1 measurements between groups both at baseline and at the end of 4 weeks treatment (table I). Duration of asthma and rate of atopy also did not differ between the groups (table I).
Comparison of eosinophil counts
and apoptotic eosinophils
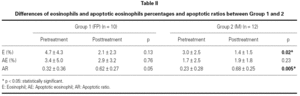
There was no difference in baseline and post treatment eosinophil percentages, apoptotic eosinophil percentages and apoptotic ratio between two groups (p > 0.05). In within group comparisons eosinophil percentage and apoptotic eosinophil percentage did not reach significant levels between baseline and posttreatment evaluations in group 1 (p > 0.05). However apoptotic ratio increased at the end of fluticasone treatment when compared with baseline in the same group (p = 0.05). The ratio of sputum eosinophils decreased significantly (p = 0.02) while there was a significant increase in apoptotic ratio (p < 0.005) in the posttreatment period when compared to baseline values in group 2 (table II).
Comparison of serum soluble Fas Ligand assay
Pretreatment sFas ligand levels were 79.6 ± 64.6 pg/ml, 45.0 ± 55.2 pg/ml and 49.8 ± 60.1 pg/ml in Group 1, Group 2 and control group, respectively. Posttreatment sFas ligand levels were 45.7 ± 62.5 pg/ml and 47.7 ± 59.5 pg/ml in Group 1 and Group 2, respectively. Serum sFasL levels were not different between the two groups both in the pre (p = 0.36) and post (p = 0.56) treatment periods. Furthermore there was no difference in serum sFasL levels between pre and post treatment periods in both groups (Group 1: p = 0.59 and Group 2: p = 0.68). There was also no difference in serum sFasL levels between the control group and study groups at baseline (p > 0.05).
DISCUSSION
In this study we have demonstrated that four weeks treatment with montelukast increased eosinophil apoptosis in induced sputum of asthmatic patients with mild persistant asthma. To our knowledge this is the first report demonstrating the effect of montelukast on eosinophil apoptosis in vivo in human mild persistant asthma. Our results suggest eosinophil apoptosis as a mechanism for the reduction of eosinophilic airway inflammation by montelukast in asthma.
Eosinophils infiltrate the bronchial mucosa of asthmatics and are detectable in bronchial tissue specimens and in sputum even during clinical remission. The overall rate of eosinophils in induced sputum was 3.79 ± 3.48 % in our study group (n = 22) with mild persistant asthma. There are few examples of eosinophil ratios from induced sputum samples of asthmatic patients. Eosinophil ratio in induced sputum of patients with mild asthma has been found to vary between 8.5-10.3 % in different studies. However there was an overlap among asthmatics grouped according to disease severity in these studies, thus there were moderate asthmatics in some study groups13,16,17. Therefore we believe that our data on induced sputum eosinophil ratios in mild persistant asthmatics is unique, as firstly the study group was a homogenous one in respect to disease severity and secondly they were patients who were not taking any antiinflammatory drug in the preeceding 12 months of the study.
Apoptosis is a physiologic form of cell death and is believed to play a key role in the resolution of inflammation. Apoptosis contributes to the resolution of eosinophilic inflammation in asthma by 1) reducing the activation of airway eosinophils and 2) facilitating their removal. In vitro studies have demonstrated that activation of inflammatory cells is reduced when they undergo apoptosis. Furthermore since the membranes of apoptotic cells remain intact, inadvertent leakage of inflammatory mediators from these cells is prevented. This is in marked contrast to cell death by necrosis, in which the cell membrane ruptures and intracellular mediators are released. This feature of apoptosis is particularly significant for eosinophils which contain cytotoxic proteins such as ECP, that are capable of damaging airway tissue. Although there are reports on the presence of apoptotic eosinophils in bronchial biopsy tissue specimens from asthmatics with different forms of severity, data on apoptotic eosinophils in induced sputum from asthmatics are very few in the literature. Recently it has been reported that apoptotic eosinophils were also detectable in cytospin slides of induced sputum from patients with seasonal allergic rhinitis2,4,10,18,19. In this study we were able to detect apoptotic eosinophils in induced sputum of patients with mild persistant asthma who were not taking antiinflammatory therapy.
Apoptotic eosinophils have been recently detected in the sputum of asthmatic patients during clinical exacerbation8. Further Foresi et al. found that the number of apoptotic eosinophils was greater in patients with symptomatic asthma compared with mild, asymptomatic disease probably reflecting the greater tissue eosinophilia in the former group18. However Vignola and coworkers have reported that the apoptotic ratio for eosinophils in bronchial biopsy tissue specimens from asthmatics tends to decrease with the increase in the asthma severity20. The ratio of apoptotic eosinophils in our untreated mild asthmatic patients (n = 22) at baseline was similar to that of symptomatic patients in Foresi and coworkers study suggesting that apoptosis operates as a controlling mechanism for eosinophilic inflammation in the airways of patients with symptomatic asthma in need of antiinflammatory treatment.
Corticosteroids have been reported to induce eosinophil apoptosis in vitro with potencies consistent with the potency of their other antiinflammatory action in normal and asthmatic subjects7,8. However in vivo data on the effects of inhaled steroids on eosinophil apoptosis is controversial. A decrease in the number of sputum eosinophils associated with an increased apoptotic index after inhaled beclamethasone treatment has been reported in patients with asthma8-10. Furthermore, Kankaanranta and coworkers reported that use of inhaled glucocorticoids reversed the delayed eosinophil apoptosis in peripherial blood in asthma21. Conversely Sano et al. demonstrated that in vitro treatment of isolated human eosinophils with fluticasone propionate caused decreased eosinophil survival and LTC4 synthesis by a mechanism not related to cellular apoptosis22. By the use of 250 mg/day of inhaled fluticasone propionate apoptotic ratio in sputum eosinophils increased approximately two fold in our patients. However the dose of fluticasone propionate was relatively low in our study compared to doses used in studies showing increased apoptosis with inhaled steroids. We believe that higher doses of fluticasone propionate could have a more significant effect on both eosinophil ratios and apoptotic index in the sputum.
Administration of leukotriene receptor antagonists results in improvement of pulmonary function and better control of asthmatic symptoms. It has also been demonstrated that leukotriene antagonists reduce eosinophils in blood by about 50 % and decrease airway eosinophils 50-60 % after 3-4 weeks treatment in addition to improving clinical parameters, but the exact mechanism underlying these effects are not fully understood23-25. We demonstrated that treatment with montelukast for 4 weeks resulted in an increase in apoptotic index of sputum eosinophils in patients with mild asthma. Our findings have relevance to the benefit derived from leukotriene receptor antagonists in the treatment of asthma. The clinical efficacy of these compounds has been attributed primarily to inhibition of leukotriene-mediated chemotaxis and bronchoconstriction. The findings in the present study suggest that inhibition of eosinophil survival by inducing apoptosis should also be considered as a potential additional mode of action of these drugs in asthma.
Fas (APO-1/CD95) is a membrane glycoprotein belonging to the tumor necrosis factor receptor family. Fas antigen is expressed on the surface of a variety of cell types, including eosinophils26. The Fas ligand is also a member of the tumor necrosis factor receptor family and induces apoptosis in Fas bearing cells. The membrane bound human Fas ligand was found to be converted to a soluble form (sFas L) by the action of a matrix metalloproteinase like enzyme27. Fas ligand/Fas receptor molecular interactions have been implicated as having an important function for the regulation of eosinophil apoptosis28. It was shown that the number of T lymphocytes and other Fas and Fasl bearing cells in the bronchial mucosa of steriod untreated asthmatic patients were not different from that of control subjects. However steroid treated asthmatics exhibited increasing expression of Fas and Fas ligand in their bronchial epithelium when compared to control subjects and untreated patients. Authors suggested that the decrease in the inflammatory cell infiltrate in the bronchial submucosa of patients treated with steroids might in part be due to the increased expression of Fas L on bronchial epithelial cells by the action steroids29. However we observed no change in serum sFasL levels following treatment with both montelukast and fluticasone in our study.
In conclusion our findings demonstrate that four weeks treatment with a Cyst LT receptor antagonist montelukast resulted in an increase in eosinophil apoptosis comparable to that of fluticasone propionate in mild persistent asthmatic patients, suggesting that induction of apoptosis may be a potential mechanism for mode of action of these drugs in asthma.
ACKNOWLEDGMENTS
We thank Duzen Laboratory Group for sFasL assays.