It has been suggested that the presence of Toxocara canis larvae in lungs is an underlying factor in the onset of asthma. Although the association of asthma and seropositivity to Toxocara has been observed, there are no studies that indicate whether these antibodies are specific against T. canis antigens.
MethodsSeroprevalence to T. canis excretion-secretion antigens (TcES Ag) were compared between asthmatic children (n=285) and non-asthmatic children (n=152), using IgG-ELISA and IgE-ELISA. The recognition patterns of TcES Ag were determined using Western blot (WB).
ResultsIgG-ELISA seroprevalence was 30.8% for asthmatic children and 19.7% for non-asthmatic children (p<0.05). IgE-ELISA seroprevalence was 7.7% for asthmatic children and 6.5% for non-asthmatic children, respectively (p>0.05). Sera of both groups positive to IgG-ELISA recognised 11 TcES Ag bands for IgG. No differences between the groups were observed regarding recognition patterns; the asthmatic group, however, presented significantly higher cross-reaction to Ascaris suum somatic antigens (AsS Ag) than the non-asthmatic group. Sixty-three sera from asthmatic children positive to IgG-ELISA were evaluated by WB for IgE and 58.7% revealed a recognition pattern for IgE. In the group of non-asthmatic children positive to IgG-ELISA, 80% presented IgE band recognition. No differences were observed between the groups regarding recognition patterns.
ConclusionsThe results observed suggest that differences in seroprevalence determined by IgG-ELISA between groups of asthmatic and non-asthmatic children reported by other authors occur because of a higher frequency of cross-reaction in asthmatic children.
Toxocara canis is the most common ascarid in dogs in Mexico, as in many other countries. Larval stages infest different paratenic hosts, including humans1, who acquire the infection by ingesting embryonated eggs of the parasite eliminated in pup faeces. The infection is more common in children with a history of geophagia (pica), deficient hygiene, and who are in close contact with dogs.
Once in the host tissue, the T. canis migrating larvae secrete molecules known as TcES Ag, which exert diverse effects on immune response cells. In mice, it has been observed that the presence of larvae in the lungs induces an increase in inflammatory cells (macrophages and eosinophils) and modifies lymphocyte subpopulations. CD4+ lymphocytes show a Th2-type response with IL-4, IL-5 and IL-10 production. The latter are responsible for the eosinophilia observed in the lungs, increased levels of total IgE, and Toxocara-specific IgG1 of infected mice2,3. IL-4 stimulates various B-lymphocyte clones, probably unspecifically, and is responsible for the production of high levels of non-specific IgG and IgE observed in human and experimental models4,5. A non-specific polyclonal activation is thus generated, which increases susceptibility to various allergens6.
It has been suggested that the presence of T. canis larvae in lungs is an underlying factor in the onset of asthma. Using ELISA, several authors have found higher frequency of antibodies against TcES Ag in asthmatic, compared with non-asthmatic, children7–9. No proof, however, has been found that these antibodies are specific against T. canis antigens. In the present study, TcES Ag seroprevalence and recognition patterns were analysed in the sera of asthmatic children in Mexico City.
Materials and methodsPatient enrollment and grouping criteriaSera of two groups of children ranging from 4 to 12 years of age were compared at the Federico Gómez Children's Hospital. The first group included sera from 285 children diagnosed with asthma and with complete medical records at the Allergy and Immunology Service of the hospital. The second group included sera from 152 children with no type of asthma and without upper or lower respiratory tract infections for a period of at least 6 weeks prior to sample collection. All children were referred from the Traumatology Service of the Hospital. Patients with incomplete medical records and those whose parents did not authorise blood sample collection were excluded. The study was approved by the Hospital Ethics Committee and all patients’ parents gave written informed consent.
A diagnosis of asthma was based on clinical symptoms and response to treatment. The severity of asthma was rated according to the symptoms and the Global Initiative on Asthma (GINA) scheme10: mild (intermittent or persistent); moderate; and severe. Lung function was measured using an EasyOne spirometer (ndd Medical Technology, Andover, Mass, USA), according to the American Thoracic Society specifications. The best test out of three technically acceptable tests was selected. Spirometric prediction equations from a Mexico city children's population were used to calculate the predicted percentage of forced expiratory volume11.
T. canis Larvae collection and cultureEgg collection and culture were performed by the Oshima12 technique. Larvae were collected and cultured by the modified techniques of Savigny13 and Bowman et al.14. Larvae were maintained in cell culture dishes (Falcon) with RPMI 1640 medium (In Vitro®), buffered with HEPES at pH 7.2 with 100μg/mL gentamicin and 1% glucose at a concentration of 104 larvae per mL. The supernatant was collected on a weekly basis and concentrated for ultrafiltration (Amicon YM-10). Protein concentration was determined by the Bradford method15.
Ascaris suum somatic antigen (AsS Ag) collectionAscaris suum adult worms were collected from autopsies of naturally infected pigs; worms were washed several times in PBS and sectioned with a knife in a sterile Petri dish. They were then homogenised in a glass Tenbrook homogeniser with 1M NaOH, and after 10min, the solution was neutralised with 1M HCl and centrifuged at 5000rpm for 1h at 4°C. The supernatant was filtered through a 0.22μm membrane, added with ether to a 1:3 ratio of the final volume, and preserved at −20°C for no more than 20 days16.
IgG and IgE ELISA for TcES AgNinety-six well polystyrene plates (Maxi sorp Nunc®) were used for this analysis. They were coated in wells with 50μL of a 1.25μg/mL solution containing TcES Ag in bicarbonate buffer (pH 9.6). Plates were washed with a PBS solution containing 0.1% Tween 20 and blocked with 3% bovine serum albumin (SIGMA®) in PBS. Sera were tested in duplicate at 1:32 dilution in PBS with 0.1% Tween 20 and incubated in the wells for 2h at 37°C. Plates were washed and incubated with a peroxidase-conjugated goat anti-human IgG antibody (Serotec®) at 1:10,000 dilution for 45min at 37°C. For the IgE ELISA: plates were coated with 10μg/mL TcES Ag, sera were tested at a 1:10 dilution, and IgE was detected with a peroxidase-conjugated anti-human-IgE polyclonal antibody (Serotec®) at 1:500 dilution for 18–24h at 4°C. Colour was developed with 0.05% OPD, 0.01% H2O2 in a citrate buffer solution and stopped after 15min with 6% orthophosphoric acid. Plates were read at a wavelength of 492nm in an Ascent ELISA plate reader (Labsystems®). Simultaneously, two control wells with no antigen were tested for each serum. The value obtained from these control wells was subtracted from the value of wells with antigen, to eliminate the non-specific colorimetric reaction17.
The IgG ELISA cut-off value was determined by the mean, plus 3 SD of the optical density (OD). The sera were taken from nine subjects negative to an ELISA-Toxocara canis kit (Alexon Trend Inc®) with no clinical history suggesting toxocariosis. Five sera from subjects positive to the same diagnosis kit were considered as the positive reference for the standardised test, all OD values being above the established cut-off value. Regarding the IgE-ELISA, a bimodal curve of prevalence was established and confidence intervals were defined with the means of the 2 populations ±2 S.D.
IgG and IgE Western blot for TcES AgTcES Ag were separated in SDS-PAGE (50μg TcES Ag per gel) at 100V, 120mA for 2h, and transferred to a nitrocellulose membrane in Transblot Semidry equipment (Bio-Rad®); the transfer was performed at 12V for 45min. The membrane was blocked in saline Tris-buffer with 3% bovine serum albumin overnight at 4°C. Subsequently, the membrane was washed 3 times in distilled water and cut into 3-mm strips. Strips were incubated for 2 h in 1:40 and 1:200 dilutions of sera, previously absorbed with AsS Ag (1mL dilution with 87.5μg AsS Ag for 30min at 37°C). Later, strips were incubated with peroxidase-conjugated rabbit anti-human IgG polyclonal antibody (Serotec®) at 1:2,000 dilution for 1h and bands were developed with a 0.05% 4-chloro-n-naphthol solution in 16% methanol with 0.001% H2O2.
Sera recognising at least 3 out of the 4 most frequently recognised antigens (51, 43, 30 and 26kD) were considered positive; some have previously been reported18. Sera presenting no specific T. canis band pattern or a pattern of only high molecular weight bands were retested at a 1:10 dilution with and without AsS Ag adsorption. Antigens which disappeared when sera were absorbed with AsS Ag, but appeared without the adsorption, were considered cross-reacting antigens.
In the IgE Western blot, membranes were blocked with the Aurora Western blot kit block solution (SIGMA®); strips were incubated for 2h in a 1:10 dilution of the sera previously absorbed with AsS Ag. Subsequently, strips were incubated with alkaline phosphatase-conjugated anti-human IgE polyclonal antibody (Sigma®) at a 1:500 dilution for 1h. Next, a chemiluminescent substrate (StarLight Aurora®) was added to the wells for 5min and stirred gently. Band detection was performed on photographic paper (Kodabrome II RC Kodak®) by contact in a radiographic cassette for 45min. The paper was developed by use of a conventional method with the manufacturer's reagents.
Statistical analysisData were analysed in contingency tables with Excel (Microsoft©) software with the Chi-square test and the Student's t-test. Linear regression was used to test the variable Optical Density mean (as the dependent variable) and number of bands recognized in the Western blot (as independent variables), linear regression analyses were performed in NCSS 2000 and PASS 2000 (Jerry Hintze©2001), All the results were considered significant p-values ≤0.05.
ResultsClinical characteristics of asthmatic populationTwo hundred and eighty-five children with asthma (184 boys and 101 girls) were recruited; average age, 6.9±2.7 years. Severity according to GINA guidelines was: mild intermittent, 63.3% (189/285); mild persistent, 21.4% (61/285); moderate persistent, 11.9% (34/285); and severe persistent, 0.35% (1/285).
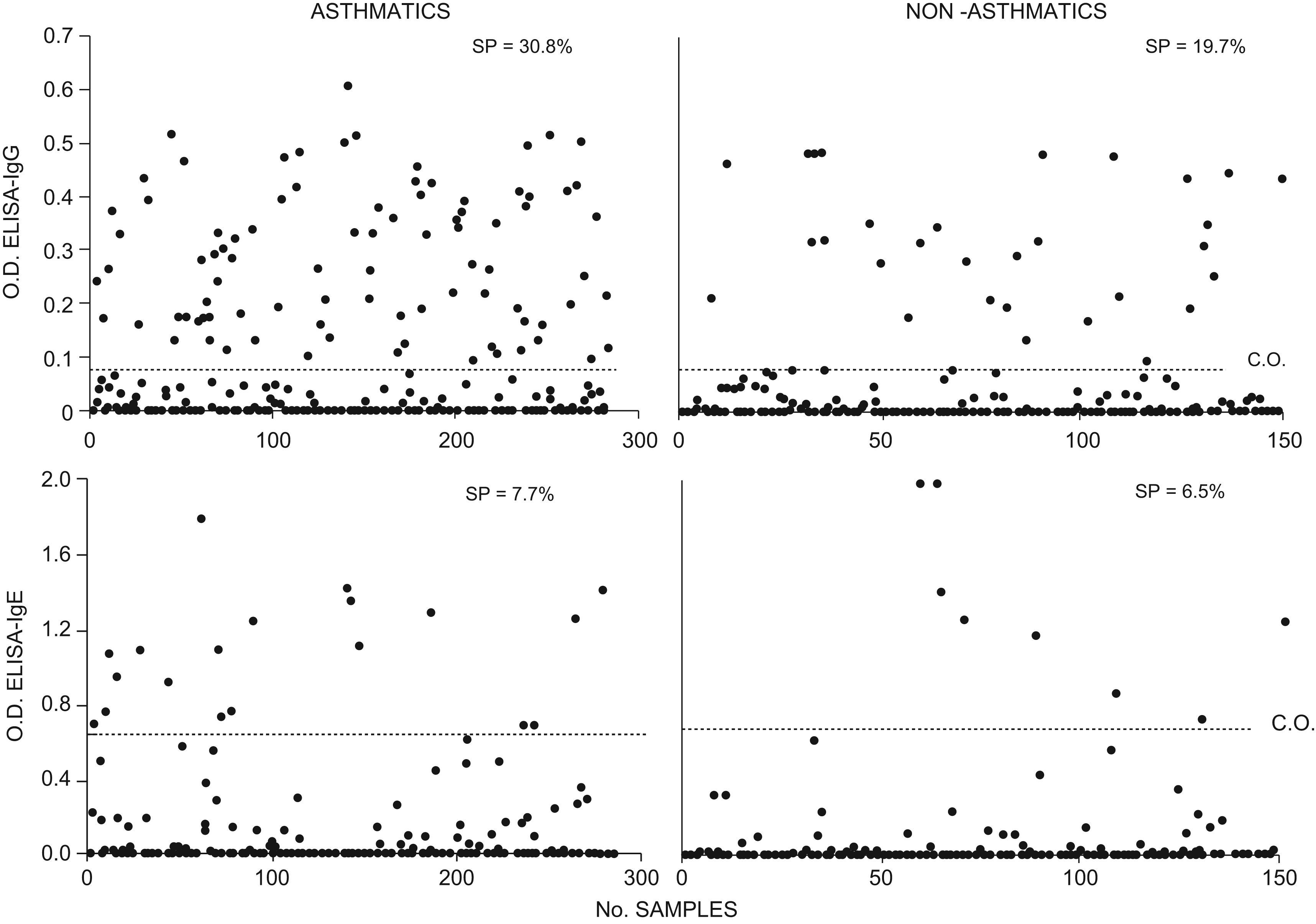
Seroprevalence by ELISAApproximately 30.8% (88/285) of children with asthma and 19.7% (30/152) of non-asthmatic children were positive to the IgG-ELISA against TcES Ag (Figure 1). These differences were statistically significant (p<0.05).
In the IgE-ELISA against TcES Ag, 7.7% (22/285) of children with asthma and 6.5% (10/152) of non-asthmatic children were positive; these data present no statistically significant difference (p>0.05). Sera positive to IgE-ELISA were also positive to IgG-ELISA (Figure 1).
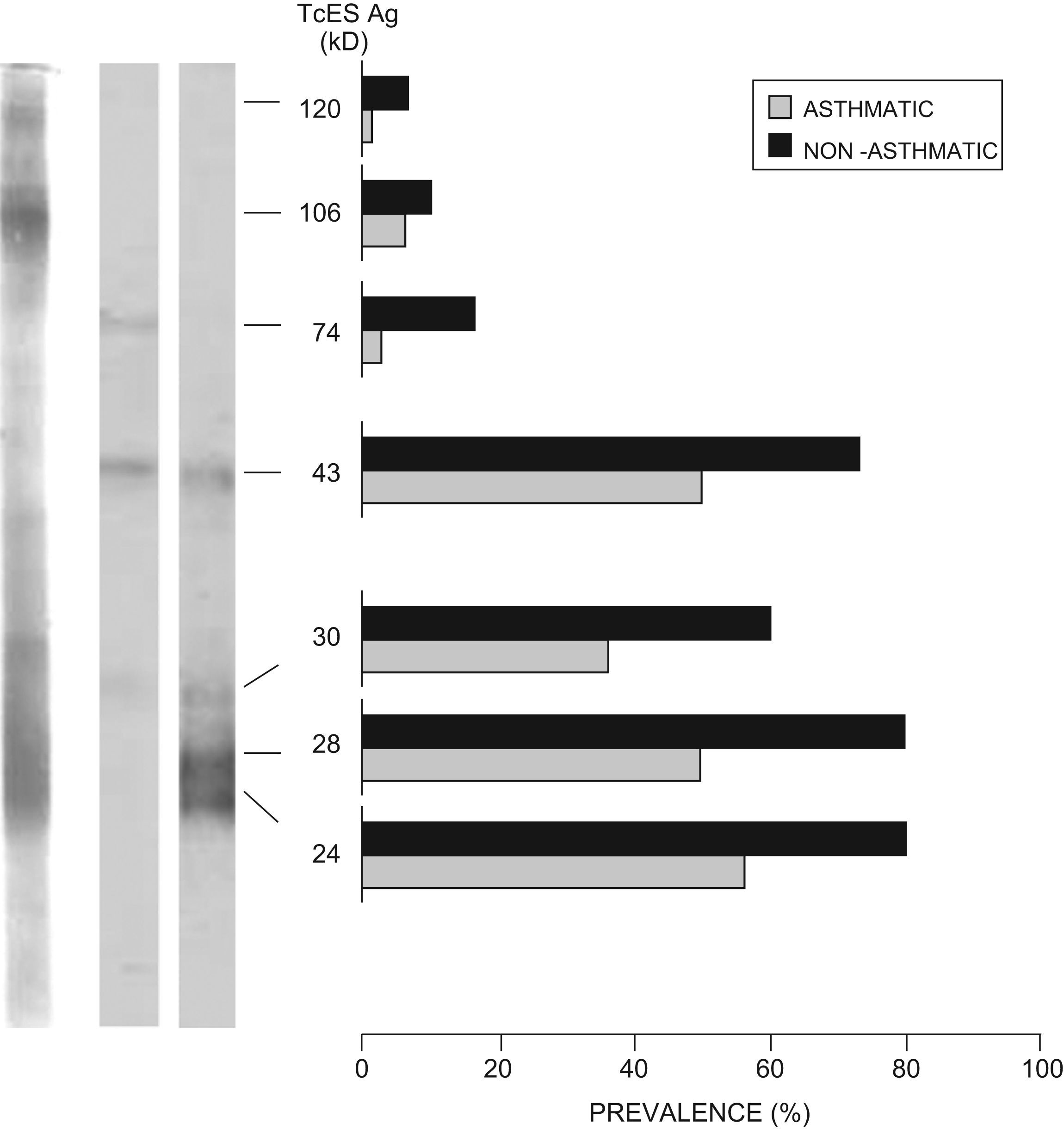
IgG-Western BlotSera from asthmatic children positive to IgG-ELISA were analysed by IgG-WB. About 62.5% (55/88) presented a positive TcES Ag band pattern (51, 43, 28 and/or 24kD). Among those positive, 16.3% (9/55) also recognised bands of higher molecular weight. A group with 10% of the sera from children negative to IgG-ELISA was randomly selected and tested by IgG-WB; none recognised TcES Ag bands. Using the same technique, sera from non-asthmatic children positive to IgG-ELISA were analysed: 86.6% (26/30) were positive to TcES Ag by IgG-WB, and 26.9% (7/26) also recognised bands with high molecular weight. Figure 2 shows antigen prevalence recognised by both groups. Significant association between the mean OD from IgG-ELISA and number of bands recognized in the IgG-WB was observed (F-ratio 60.2, p<0.001).
IgE-Western BlotSera from asthmatic children positive to IgG-ELISA were analysed by WB-IgE; 59% (52/88) recognised TcES Ag bands. In non-asthmatic children positive to IgG-ELISA, 83.3% (25/30) recognised TcES Ag bands by WB-IgE. Figure 3 shows antigen prevalence recognised by both groups. Significant association between the mean OD from IgE-ELISA and number of bands recognized in the IgE-WB was observed (F-ratio 26.2, p<0.001).
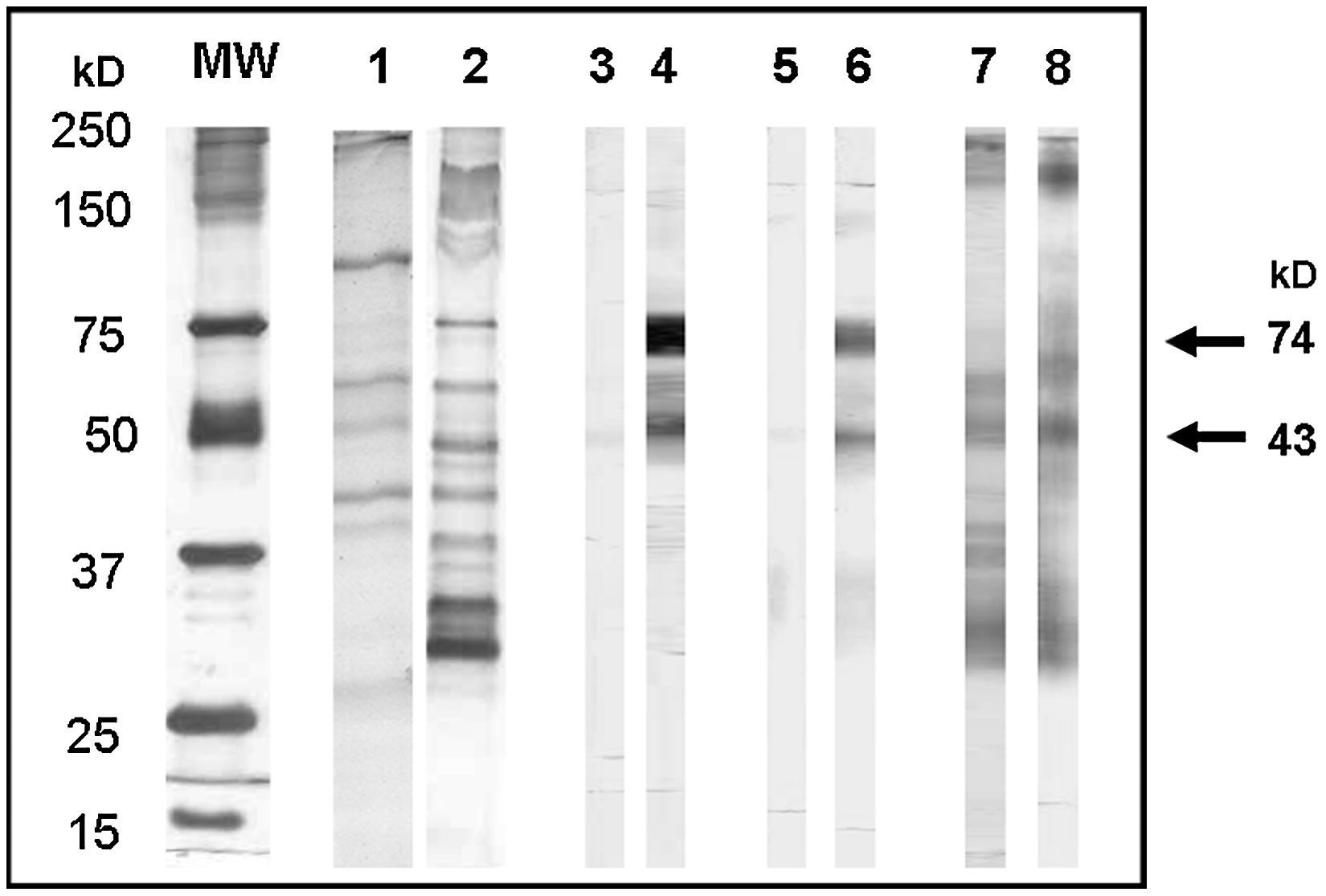
Cross-reacting antigensApproximately 75.7% (25/33) of the sera from asthmatic children positive to IgG-ELISA, which did not recognise any band in the IgG-WB with AsS Ag adsorption, recognised antigens of 74kD (25/33), 43kD (15/33), and 51kD (2/33) when the WB was performed with sera non-absorbed with AsS Ag. These were considered cross-reacting antigens (Figure 4); 24.2% (8/33) did not recognise any band.
Toxocara canis excretion-secretion antigens (TcES Ag) recognised by sera from asthmatic (sa) and from non-asthmatic children (sna) positive to IgG-ELISA with and without adsorption with somatic antigens of Ascaris suum. Lane 1, SDS-PAGE of AsS Ag; lane 2, SDS-PAGE of TcES Ag; lane 3, WB sa with adsorption; lane 4, WB sa without adsorption, lane 5, WB sna with adsorption; lane 6, WB sna without adsorption; lane 7, WB sa positive with adsorption; lane 8, WB sna positive with adsorption; MW, molecular weight markers. Cross-reacting antigens are indicated by arrows.
About 25% (1/4) of the sera of non-asthmatic children, negative to IgG-WB with AsS Ag adsorption, but positive to IgG-ELISA, recognised only cross-reacting bands with AsS Ag, and 75% (3/4) did not recognise any band.
DiscussionAsthma is a disease characterised by inflammation of the respiratory tract associated with hypersensitivity, producing respiratory symptoms and reversible episodes of lung function reduction19. Factors predisposing to this disease include those that cause tissue damage and local immune alterations. It has been demonstrated that Toxocara canis is a nematode that migrates erratically in the host lungs producing lesions, irritation, and a strong inflammatory reaction6, therefore suggesting that the presence of this parasite is a predisposing factor in the onset of asthma.
In the present study, Mexican asthmatic patients presented higher seroprevalence (p<0.05) of IgG antibodies against TcES Ag than the group of non-asthmatic children (30.8% and 19.7%, respectively). Similar differences have previously been found by other authors: Buijs et al.8 found a significantly larger number of asthma cases and recurrent bronchial issues in a group of children with high anti-T. canis IgG antibody titres in the United States; Lokman et al.9 reported values of 57.8% and 15.4% for asthmatic and non-asthmatic children, respectively, in Malaysia.
The IgE-ELISA test did not reveal significant differences (p>0.05) in seropositivity to TcES Ag between asthmatic and non-asthmatic children (7.7% and 6.57%, respectively). Buijs et al.8 reported a significant increase of serum IgE to environmental allergens in seropositive subjects, but the amount of IgE specific to TcES Ag was not determined.
It has been demonstrated that ascariosis is the parasitosis most frequently associated with toxocariosis, mainly because the epidemiological conditions required for infection in these parasitoses are quite similar20. Some studies have reported that somatic antigens of T. canis antigenically cross-react with other helminths21. These cross-reactions significantly decrease when TcES Ag are used in the serodiagnosis15; some cross-reaction may, however, be present with antigens of other ascarids. To reduce this cross-reaction, adsorption of test sera with soluble extracts of Ascaris lumbricoides or Ascaris suum have been used22. In the present study, antigens recognised by WB after serum adsorption with AsS Ag were considered specific to T. canis; four of these antigens (24, 26, 30 and 35kD) corresponded to those described as specific by Magnaval et al.18. Antigens that were recognised when the same sera were tested by WB, without AsS Ag adsorption, were considered cross-reacting antigens.
A higher percentage of non-asthmatic children positive to IgG-ELISA recognise antigens specific to TcES Ag, in comparison with asthmatic children (86.6% and 62.5%, respectively). However, the antigens’ cross-reacting recognition percentage was greater (p<0.05) in the group of asthmatic children (28.4%), compared with the group of non-asthmatic children (10%). These data reveal that asthmatic children have a higher tendency to present cross-reaction among antigens, which suggests that differences found in IgG-ELISA in favour of asthmatic children in this and other studies are the result of a higher incidence of cross-reaction in this group. On the other hand, these results may also indicate that asthma is facilitated by a combination of parasitic infections in which other helminths, apart from T. canis, may be involved.
There are no reports regarding differences in frequency of recognition of each TcES Ag in the asthmatic and non-asthmatic children's groups. This is significant, given the possibility that differences in the seroprevalence of these groups, reported by other authors, are due to differences in the TcES Ag immunodominance of each group and, consequently, differences in recognition by IgG or IgE. Results obtained in the IgG-WB revealed that recognition frequencies of TcES Ag do not show differences between the groups, but are clearly homogeneous. These results indicate that there are no differences between asthmatic and non-asthmatic children regarding the immunodominance of the TcES Ag that induce the IgG.
Differences in the recognition of antigens stimulating the IgE response (allergenic antigens) were also analysed in the present study. Previously, Takamoto et al.23 observed (in lung cell cultures of nu/nu mice infected with T. canis) that non-specific polyclonal response by IgE is caused by the secretion of IL-4 and IL-5 by lung cells, further characterised as CD4- and CD8- T lymphocytes (double negative). A toxocariosis and allergic asthma model in mice revealed that BALBc mice are more prone to asthmatic manifestations than C57BL/6 mice when infected with T. canis. Furthermore, they are sensitised by a respiratory path with ovalbumin24, indicating that there is a genetic element in the development of asthma triggered by T. canis infection. These studies have been performed with total TcES Ag, and experimental infection, respectively. In the present study, no differences were found in the recognition pattern by IgE between the asthmatic and non-asthmatic children's groups. The higher IgG seroprevalence to TcES Ag found in asthmatic children, in comparison with non-asthmatic children, can therefore be explained by cross-reaction with other helminths and not necessarily by a T. canis infection.
Differences in the ELISA and Western blot results in the present work, could indicate the importance of the sera pre-absorption with Ascaris sp antigens as a more adequate way for serological Toxocara diagnosis, as suggested by other authors25,26; the previous observation could be particularly important within the Toxocara seropositive asthmatic patients, based on the results presented in this work, where the specific recognition of antigens was improved by the pre-absorption of the sera tested by Western blot in contrast with the results presented by other authors8,9,27,28. Additionally, most of the commercial Toxocara diagnosis kits do not use the sera pre-absorption step, therefore this is probably reflected in an overestimation of the true seroprevalence.
We deeply thank César Cuenca Verde and Alejandra Ayanegui Alcérreca for technical assistance. This study was supported in part by a grant from the Programa de Apoyo a Proyectos de Investigación e Inovación Tecnológica (PAPIIT-UNAM).