Ataxia-telangiectasia (A-T) is a rare and degenerative disease that leads to varying degrees of immunodeficiency, oxidative stress, and malnutrition. Vitamin A and zinc are essential for immune function and antioxidant defence.
ObjectiveTo compare levels of retinol, beta carotene, and zinc in patients with ataxia-telangiectasia and healthy controls.
MethodsWe performed a cross-sectional study with 14 AT patients and 14 healthy controls matched for age and gender. All participants underwent a nutritional and laboratory evaluation comprising concentrations of retinol, beta carotene, serum and erythrocyte zinc, malondialdehyde (MDA), T lymphocyte numbers (CD4+ and CD8+) and immunoglobulin (IgA).
ResultsThe AT patients showed high rates of malnutrition with reduced lean body mass when compared to the control group. However, the concentrations of MDA, retinol, beta carotene, and serum and erythrocyte zinc in AT patients were similar to those of the control group. The retinol levels presented a negative correlation with MDA and positive correlation with IgA serum level.
ConclusionsThe AT patients assessed showed no change in nutritional status for vitamin A and zinc; however, they presented severe impairment in overall nutritional status observed and correlation between retinol with MDA and IgA.
Ataxia-telangiectasia (AT) is a rare degenerative disease that affects approximately 1 in 40,000 liveborn infants.1 This disease has an autosomal recessive inheritance and progresses with neurodegeneration, immunodeficiency, sinopulmonary infections, telangiectasia, predisposition for malignancy, radiosensitivity, premature aging and over 50% of the patients suffer from malnutrition.2,3
The gene which is defective in this disease, ATM (ataxia-telangiectasia-mutated), codes for a protein kinase that participates in multiple signal-transduction pathways. The ATM-deficiency induces oxidative stress, which is potentiated by the constant presence of infections and leads to cell death by apoptosis.4
Oxidative stress results in a reduction in the plasma antioxidant defence and reduced plasma concentrations of vitamins A and E, which leads to an increase in the level of lipid peroxidation biomarkers and oxidative damage to the DNA of leucocytes.5,6
Some micronutrients such as vitamin A and zinc are essential for the maintenance of immune function and antioxidant defence. Zinc is essential for the development and function of neutrophils, macrophages, and natural killer cells. Thus, zinc deficiency leads to the reduction of thymulin, interleukin-2, and interferon-gamma, increases production of pro-inflammatory cytokines and is associated with a higher incidence of infections.7,8 Zinc also participates in antioxidant defence by inhibiting NADPH oxidase, acting as a structural and catalytic component of the enzyme superoxide dismutase and inducing the synthesis of metallothionein.7
Vitamin A, particularly in its retinoic acid form, plays a number of roles in adaptive immunity, which include the activation and proliferation of T cells, production of interleukin 2, inhibition of B cell apoptosis, modulation of antigen presentation, regulation of the Th1–Th2 balance, differentiation of regulatory T cells and the production of immunoglobulin A (IgA). Vitamin A deficiency is associated with a decrease in the intestinal immune response and an increased risk for the development of gastrointestinal and respiratory infections.9
The few studies that have assessed the nutritional status of patients with AT have reported that this disease is correlated with changes in the body mass index (BMI)10 and in the serum concentrations of retinol.5
The aims of the present study are to evaluate the nutritional status, to measure the concentrations of retinol, beta carotene, serum and erythrocyte zinc in the plasma of AT patients, and also to investigate the relationship between these micronutrients with malondialdehyde levels, the number of T lymphocytes (CD4+ and CD8+), and the serum and secretory IgA levels.
Patients and methodsThe present study is a controlled cross-sectional study involving 14 AT patients, aged 3–20 years, who were diagnosed with AT according to the diagnostic criteria by the European Society for Immunodeficiencies (ESID). Nine of them (64.2%) were receiving regular infusions of intravenous immunoglobulin, and eight (57.1%) were regularly taking antibiotics. None of the patients had acute infections at the time of sample collection.
The control group consisted of 14 age- and sex-matched healthy individuals.
A questionnaire was used to assess each patient's demographic, socioeconomic, and clinical issues and the study was approved by the Research Ethics Committee.
Anthropometric evaluation and food consumptionThe anthropometric evaluation included the measurement of weight, height, and skinfold thickness (tricipital and subscapular), which were measured according to the World Health Organization (1995).11
The patients who were unable to stand upright were weighed in their parent's arms and their recumbent height was measured on a firm, flat surface using an inextensible tape that was graduated in millimeters.
Body mass index (BMI) and height to age ratio (H/A) were expressed as Z scores. The values recommended by the World Health Organization were used as reference.12
The body composition was estimated using the equation based on the sum of the tricipital and subscapular skinfolds.13 The classification of the percentage of fat body was performed according to Deurenberg et al.14 The fat body mass and lean body mass were also calculated in kilograms (kg).
The stage of pubertal development was assessed based on the criteria proposed by Marshall and Tanner.15
The assessment of food consumption was performed using a 24-h dietary recall,16 which was requested on two separate occasions with an interval of 30 days between recalls.
The calculation of the nutrients in the diet of a patient was performed with the help of the Diet Win program.17 The total energy consumption, the intake of proteins per kg of body weight, and the levels of retinol, beta carotene, and zinc were assessed and compared between the patients and the controls.
Biochemical assessmentAfter an 8-h fast, 20mL of blood was collected in a dimly lit room and the levels of retinol, beta carotene, serum and erythrocyte zinc, malondialdehyde (MDA), serum IgA, and CD4+ and CD8+ T lymphocytes were determined. Saliva samples, which were obtained in the absence of stimulation, were collected to determine the levels of secretory IgA.
- •
The retinol and beta carotene levels were determined by high-performance liquid chromatography (HPLC).18,19
- •
The serum and erythrocyte zinc levels were measured using atomic absorption spectrophotometry (“Perkin Elmer” model 5.100).20
- •
The malondialdehyde level was assessed by spectrophotometry21 using reference values of 1.0–3.5nmol MDA/mL.
The statistical analyses were performed using the Minitab® (version 15.1) and Bioestat® (version 5.0) programs. Student's t-test and the Mann–Whitney test were used to assess the difference between the quantitative variables. The Chi-squared and Fisher's exact tests were used to determine the association between the qualitative variables. The Pearson's correlation coefficient and Spearman's correlation coefficient were used to determine the correlations. A 5% level of statistical significance was considered (p<0.05).
ResultsPatients and control features are shown in Table 1. Both groups were similar in terms of age, pubertal development, income per capita, and maternal education (Table 1).
Characteristics and nutritional status of patients with ataxia telangiectasia (AT) and controls. Mean±SD; median (min–max).
| Variables | Patients (n=14) | Controls (n=14) | p-Value |
| Age (years) | 13.1±4.96 | 13.2±4.81 | 0.877a |
| Males | 11/14 (78.57%) | 11/14 (78.57%) | |
| Pubertal development | |||
| Pre-pubertal | 6/14 (42.8%) | 4/14 (28.5%) | |
| Pubertal | 2/14 (14.2%) | 6/14 (42.8%) | 0.836b |
| Post-pubertal | 6/14 (42.8%) | 4/14 (28.5%) | |
| Income per capita/month ($) | 239.45±185.40 | 311.35±138.37 | 0.255a |
| Maternal education (years) | 10.5 (1–12) | 7.5 (0–12) | 0.448b |
| Nutritional status | |||
| BMI (kg/m2) | 15.41 (12.7–22.5) | 18.3 (14.9–20.7) | |
| Malnourished | 6/14 (42.8%) | 0 | 0.009c,* |
| Eutrophic | 7/14 (50.0%) | 12/14 (85.7%) | |
| Overweight | 1/14 (7.1%) | 2/14 (14.3%) | |
| Height (cm) | 139.5 (97–163) | 155.5 (119–183) | |
| Low height | 5/14 (35.7%) | 0 | |
| Body composition | |||
| % body fat | 16.93 (12.6–32.9) | 15.2 (10.6–25.7) | 0.550b |
| Adequate | 11/14 (68.6%) | 11/14 (68.6%) | |
| High | 3/14 (21.4%) | 2/14 (14.3%) | |
| Low | 0 | 1/14 (7.1%) | |
| Fat body mass (kg) | 6.27±3.61 | 7.42±3.07 | 0.369a |
| Lean body mass (kg) | 25.55±9.27 | 37.60±11.8 | 0.006a,* |
A higher percentage of short stature and malnutrition with reduced lean body mass (kg) was observed in the patient's group when compared with the control p<0.05 (Table 1).
The concentrations of micronutrients and MDA were similar between the patients and the controls (Table 2). However, the MDA values were above the reference value (3.5nmol MDA/mL) in 28.5% (4/14) of the patients. MDA and retinol levels were similar between patients with and without immunoglobulin replacement (p=0.774 and p=0.224 respectively) and also when we compared patients with and without antibiotics (MDA p=0.404 and retinol p=0.744).
Serum concentrations of malondialdehyde (MDA), retinol, beta carotene, and serum and erythrocyte zinc in patients with AT and controls. Mean±SD; median (min–max).
| Patients (n=14) | Controls (n=14) | p | |
| MDA (pg/mL) | 3.12±0.69 | 2.65±0.56 | 0.063a |
| Retinol (μmol/L) | 1.65 (1.0–4.2) | 2.00 (0.9–3.4) | 0.241b |
| Beta carotene (μmol/L) | 0.25 (0.10–1.70) | 0.20 (0.10–0.40) | 0.129b |
| Serum zinc (μg/dL) | 125 (100–150) | 150 (100–150) | 0.112b |
| Erythrocyte zinc (μgZn/gHb) | 43.32±9.89 | 44.86±8.37 | 0.660a |
Seven (50%) patients were on regular use of vitamin supplements but no association was found between this supplementation and the concentrations of assessed micronutrients (p>0.05).
There was a significant negative correlation between the concentrations of MDA and retinol in the group of patients (r=−0.628, p=0.016), which was not observed in zinc and beta carotene (Table 3).
Correlation between the concentrations of malondialdehyde (MDA), retinol, beta carotene, and serum and erythrocyte zinc in AT patients and controls.
There was not a significant association between the nutritional status, the body composition, and the concentrations of micronutrients in the patients and controls (p>0.05).
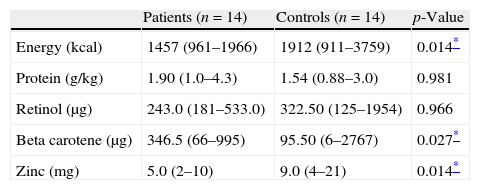
The median values of energy (p=0.014) and zinc (p=0.014) consumption were lower in patients when compared to the controls (Table 5).
Energy, protein, retinol, beta carotene, and zinc consumption in AT patients and controls. Median (min–max).
| Patients (n=14) | Controls (n=14) | p-Value | |
| Energy (kcal) | 1457 (961–1966) | 1912 (911–3759) | 0.014* |
| Protein (g/kg) | 1.90 (1.0–4.3) | 1.54 (0.88–3.0) | 0.981 |
| Retinol (μg) | 243.0 (181–533.0) | 322.50 (125–1954) | 0.966 |
| Beta carotene (μg) | 346.5 (66–995) | 95.50 (6–2767) | 0.027* |
| Zinc (mg) | 5.0 (2–10) | 9.0 (4–21) | 0.014* |
Mann–Whitney test.
The mean number of CD4+ T lymphocytes in the patients was 408.4±157.6/mm3, and 92.8% (13/14) of these patients exhibited values below the 10th percentile for their age group. The mean number of CD8+ T lymphocytes was 396.4±298.9/mm3; half (50%, 7/14) of the patients had values below the 10th percentile. There was no correlation between the number of T lymphocytes and the concentrations of micronutrients (p>0.05) (Table 4).
Correlation between the number (mean) of T lymphocytes (T CD4+ and T CD8+) and retinol, beta carotene, and serum and erythrocyte zinc in AT patients.
Nine patients (64.2%) presented IgA levels<7mg/dL, and 40% (4/10) of the patients had salivary IgA<0.01mg/dL. There was a significant positive correlation between serum IgA levels and plasma retinol (p=0.015, r=0.633).
DiscussionThe present study showed that patients with AT exhibit high rates of malnutrition, short stature and reduced lean body mass, which is in agreement with Schubert et al.,10 who found that a high percentage (58%) of AT patients had a body mass index below the third percentile despite adequate energy intake. These authors also observed a progressive reduction in the BMI with increasing age.
Numerous factors can explain the impaired weight and height in AT patients such as neurodegeneration, limited food intake, dysphagia and/or swallowing incoordination, limited physical activity, and hormonal changes, hypogonadism, insulin resistance, glucose intolerance, abnormal expression of IGF1 (somatomedin C), and low levels of IGFBP3 (insulin-like growth factor binding protein-3).10,22,23 Nine from 14 patients were in wheelchairs which naturally reduces the physical activity and consequently food intake. All presented mild dysphagia. Caring about the nutrition in these patients is extremely important as increased morbidity and mortality are associated with nutritional status and immune function.24,25 For some AT patients gastrostomy is recommended resulting in an improvement in the quality of life26 but none of ours were submitted to it.
An anthropometric assessment based on simple measurement, such as weight and height, is insufficient to identify changes in body composition. Compromised lean body mass is associated with a worsening prognosis in patients with chronic diseases, such as congestive heart failure.27 An accurately estimated body composition will prove beneficial for guiding the nutritional therapy of patients with AT.
The retinoic acid (RA) activates ATM and this does not involve DNA damage.28 It was previously shown that AT patients exhibit lower concentrations of retinol5 in contrast to our findings, although the retinol level was determined by the same method (HPLC). The age for both groups was similar so the different outcomes may be explained by a number of factors, such as the improvement in treatment protocols, including multi-professional care and a greater awareness of the importance of nutritional status. Another possibility is that the groups are different in relation to genotype.29 Patients with other immunodeficiencies, such as the common variable immunodeficiency (CVID) and the acquired immunodeficiency syndrome (AIDS), also exhibit reduced concentrations of micronutrients when compared to healthy controls.30,31 This difference is most likely to be due to the higher frequency of infections and gastrointestinal impairment that affect the immunodeficient patients.32,33
Evidence has emerged that AT cells exist under a constant state of oxidative stress with high levels of reactive oxygen species (ROS), which in turn may contribute to numerable degenerative processes observed in the disease.34 Increased levels of MDA (a lipid peroxidation marker) have also been described in AT patients.4,35 Although there was no statistical difference in MDA levels between groups, we observed a tendency higher levels in the patient group (3.12±0.69) compared to controls (2.65±0.56), (p=0.063) and 28.5% of the patients presented MDA levels higher than normal. These factors might explain the significant inverse correlation between MDA and retinol in the patients group. Like carotenoids, the retinol in its antioxidant activity inhibits the transcription of the iNOS gene, a compound that stimulates the production of other free radicals, especially nitric oxide (NO).36
It is also possible that the adequate concentrations of vitamin A and zinc contributed to our results because these micronutrients have antioxidant activity and are associated with peroxyl radicals, which are capable of propagating lipid peroxidation in cells and generating hydroperoxides.7,37
A compromised mucosal immunity is a well-established hallmark of vitamin A deficiency. Retinoic acid, which is a compound derived from vitamin A, upregulates the expression of gut-homing receptors, including α4β7-integrin and CC chemokine receptor 9 (CCR9), which are important for the migration of IgA class-switched B cells.38 Gut-associated dendritic cells increase the homing of B and T lymphocytes to the small intestine in the presence of physiological doses of all-trans retinoic acid (atRA). In addition, the dietary depletion of vitamin A decreases the numbers of IgA-secreting B cells and T cells in the lamina propria.39,40 The appropriate concentration of retinol in these patients, coupled with the high frequency of serum and secretory IgA deficiency, demonstrates that this deficiency is intrinsic to the disease and not dependent on the plasma concentration of retinol. Another possibility is that these patients require a higher quantity of retinol to show any change in secretory IgA levels.
Furthermore, no correlation was found between the number of CD4+ and CD8+ T lymphocytes with the concentrations of micronutrients, which reinforces the hypothesis that the immunodeficiency is associated with the ATM protein.41 A reduced diversity of the antigen-receptor repertoire of TCR in AT patients was demonstrated and this restriction could be more dependent on constraints of TCR generation rather than a generalized decrease of thymopoiesis.42 Almost all of our patients had low numbers of CD4+ T lymphocytes and a decreased response to pneumococcal vaccine,43 which underlines the recommendation for regular infusions of intravenous immunoglobulin.
The AT patients assessed showed no change in nutritional status for vitamin A and zinc; however, they presented severe impairment in overall nutritional status. In order to keep an adequate nutritional status it is important to stimulate food containing antioxidant vitamins and oligoelements. There is no formal recommendation to supplement AT patients routinely but we have to keep in mind that vitamin A can improve the immunoglobulin synthesis in immunodeficient patients44 and anti-oxidant such as N-acetyl-l-cysteine showed beneficial effects particularly, reducing lymphomagenesis in Atm deficient mice45 reinforcing the importance of nutrition in these patients.
Considering the impact of micronutrient homeostasis on the immune and nervous systems and the correlation between retinol with MDA and IgA, appropriate monitoring of these factors and adequate nutritional interventions should be considered as part of the treatment.
Ethical disclosuresProtection of human subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Financial supportThis project received financial support from FAPESP (Foundation for Aid to Research of the State of Sao Paulo), project number 08705-5/2008.
Conflicts of interestNothing to declare.