Asthma is a heterogeneous disease characterised by chronic inflammatory airways, and is affected by several immunological factors. One of the most discussed and researched hypotheses is the relationship between vitamin D serum levels with asthma. This study aimed to investigate the relationship between vitamin D serum levels with asthma and pulmonary functions in children in Kurdistan province, Iran.
Materials and methodsIn this case–control study, 120 children ranging from 6 to 18 years were referred in summer for investigation. Participants were divided into two groups: asthma group, N=60; and control group, N=60. After serum separation, samples were analysed using vitamin D ELISA kit. Additionally, pulmonary function test and serum IgE levels were measured in both groups. Data were analysed using Chi-square test and multiple regression analysis in SPSS15.
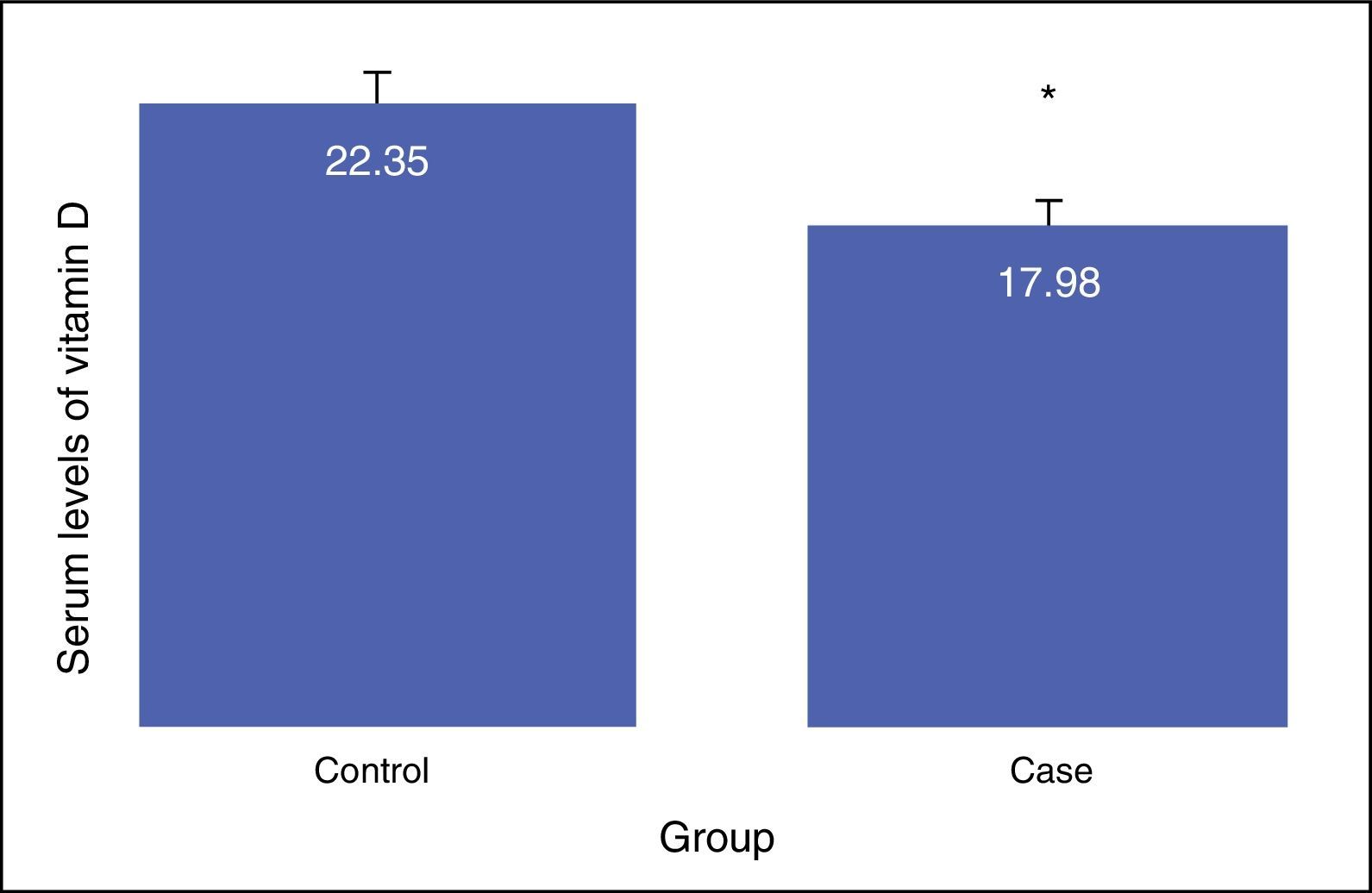
ResultsNo difference was shown between the groups in terms of gender composition (male: 57.5; female: 42.5) (P>0.05). Average Vitamin D serum level in the case group (17.98±8.68) was less than in control group (22.35±6.26) (P<0.01). In addition, the difference of vitamin D deficiency in level of suboptimal between the asthma (17.77±6.41) and the control group (24.9±3.18) was statistically significant (P<0.0001). Positive correlation existed between vitamin D levels with FEV1, FVC, and FEV1/FVC. Multiple regression analysis showed a reverse relationship between vitamin D levels with IgE serum levels; this remained after adjustment for potential confounders (e.g. age, sex, BMI, FEV1, and FVC).
ConclusionThe results showed that serum levels of vitamin D in asthma patients were less than in healthy people, and also reduced lung function in these patients. So, the serum levels of vitamin D in asthma patients must be checked continuously.
Asthma is a heterogeneous disease that is characterised by chronic inflammatory airways. Asthma is a disease that has a high prevalence in developed and developing countries. Some countries such as in the West, have a population of more than 1.3% of children that are involved in asthma and allergies.1,2 There is an estimation that about 400 million people will be suffering from asthma up to 2025 in the world.3
A change in lifestyle, diet, allergens, various infections, and the family history can be risk factors for asthma. Asthma is formed by many immunological factors. One of the most discussed and researched hypotheses is the relationship between serum vitamin D levels and asthma. Vitamin D is a fat-soluble vitamin that is made mainly in the epithelial cells of the skin, which occurs due to UV rays and the conversion of 7-dehydrocholesterol to vitamin D.4 An active form of vitamin D has local effects due to infections, and might reduce the inflammation that became a cause of those infections.5 It seems that in order to respond to an infection, vitamin D participates in Toll-like receptor signalling by up-regulating the production of cathelicidin and other antimicrobial peptides.6
The high incidence of vitamin D deficiency has led to more attention than in previous years, according to the report of the Centres for Disease Control and Prevention, the percentage of adults that have an adequate amount of vitamin D (as defined by 25(OH)D of at least 30ng/ml), from 60% in 1988–1994 reached almost 30% among white people and 5–10% in African Americans during 2001–2004.7 Vitamin D deficiency in the world is epidemically prevalent, and in countries surrounding the Persian Gulf, despite adequate sunlight, vitamin D deficiency has the highest prevalence. Studies have shown that vitamin D deficiency in adolescent girls in Iran and Saudi Arabia is over 70% and over 80%, respectively.8
Vitamin D affects the innate and adaptive immune systems and the regulations of Th1/h2 and phagocytosis.9 By inhibiting the production of Th17, vitamin D can also help in preventing asthma exacerbations.10 Vitamin D is considered as a potent modulator of the immune system11 that regulates cell proliferation and differentiation.12 It is assumed that vitamin D changes the expression of smooth muscle's chemokines in the airways through the inhibition of the expression of steroid resistant gene. However, this theory has yet to be proven.
Some studies have clearly shown a correlation between vitamin D deficiency and asthma,13–15 but others have provided different results.16 An inverse association between serum Vitamin D levels and the recent upper respiratory tract infections, especially in a chronic respiratory disease such as asthma, was found by Ginde et al.17 One of the studies has shown that the intake of vitamin D supplementations from childhood might be related to the risk of developing asthma or other chronic diseases.16 On the other hand, a case–control study presented that serum vitamin D levels did not differ between the asthmatic patients and the controls.18
In addition, the amount of vitamin D that might be needed to prevent or reduce the severity of an asthma attack is still unknown. Given the vital role of vitamin D in the body, and the high prevalence of asthma, as well as a lack of vitamin D, we decided to conduct a case–control study in order to investigate the relationship between serum levels of vitamin D with asthma and lung function.
Materials and methodsThis case–control study investigated the 6–18 year-old children that were referred in the summer (May–September) to the tertiary referral hospital in Kurdistan province, Iran. The study population comprised 120 people divided into two groups of 60 people: case (asthmatic) and control groups. They were selected using a convenience sample method. This study was approved by the ethics committee in Kurdistan University of Medical Sciences (ethical code: MUK.REC.1393.106). With type I error of 5%, and the power of the study of 95%, the average vitamin D in the case and control groups was 49.29±21.44 and 66.82±28.76, respectively,19 A required sample size of 56 people in each group was calculated. In this study, 60 people were obtained in each group.
Inclusion criteriaCase group: Patients with asthma, who were diagnosed by a physician with a fellowship degree in allergy and clinical immunology based on a history and physical examination and findings of pulmonary function test (PFT), and were willing to participate in the study and who had no previous known diseases. These patients were selected from the asthma and allergy clinic in Besat hospital. Initially, all patients underwent a spirometry (Spiro lab III, Italy), and the spirometry findings were used to confirm and determine the severity of the disease. ATS criteria was used to interpret the spirometry findings.
Control group: Healthy people from the community, who had no known history of asthma, no known illness, or drug use or other allergic diseases in themselves or their first degree relatives until then (in other words, healthy in every respect) and had desire to participate in the study.
Exclusion criteria included a persons’ unwillingness to continue to cooperate in the study; recognition of any impairment or chronic illness during the study such as kidney or liver disease; the risk of malignancies; known musculoskeletal disorders, including rickets and osteomalacia; the use of vitamin D as a drug or dietary supplement; the use of medications with potential impact on vitamin D metabolism such as anticonvulsants; and/or identify any diseases associated with vitamin D in the study populations, such as thyroid and parathyroid glands, disorders, acute and chronic digestive disorders, autoimmune diseases, chronic kidney disease and skin disorders.
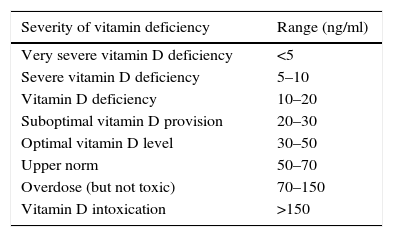
Data collection methodA sampling was taken from a person in a laboratory, and subsequently the sample was tested in the laboratory after the serum was separated under the supervision of a pathologist or a specialist in laboratory science by vitamin D ELISA assay Kit (Immunodiagnostic EIA; Bensheim and Biomedica, Wien, Austria) and was explained by observing the following values20:
Pulmonary-function testingIn both groups (case and control), spirometry was performed in accordance following the American Thoracic Society guidelines, in the seated position without nose clips. After at least one practice blow, three FEV1 and FVC readings were recorded. The highest FEV1 and FVC values from satisfactory manoeuvres were used in the analyses.
Serum total immunoglobulin EA quantitative analysis of IgE was performed using ELISA kit (Pishtaz Teb, Iran) according to the manufacturer's guidelines.
Ethical considerationsAll moral codes regarding the study and the confidentiality of information were observed, and also an informed consent form was obtained from the subjects. No additional processes were performed on the patient.
Statistical analysisDescriptive analyses were performed to calculate frequency distributions and Pearson correlation coefficients were computed to assess the association between vitamin D and other independent variables. The chi-square test and T-test were performed for categorical and continuous variables, respectively.
Hierarchical multiple linear regression model was used to examine the relationship between vitamin D and age, and sex and ige. Models were generated in three stages: model 1, did not adjust for confounders, model 2 adjusted for FEV and FVC and model 3 further adjusted for BMI. The SPSS15 statistical software was used to conduct all analyses. All tests were two-tailed and a probability value P<0.05 was considered statistically significant.
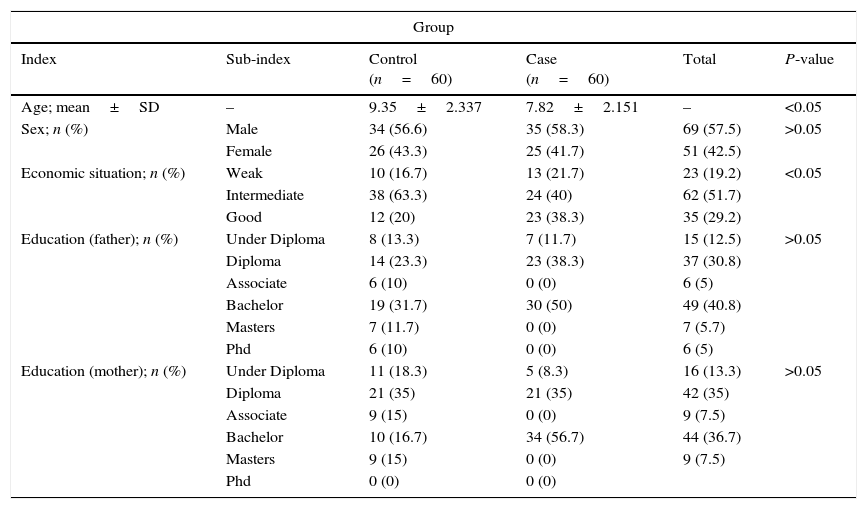
ResultsIn this study, 120 children were divided into two groups of 60: healthy children 6–18 years old (control group) and 60 children with asthma (the case group). Of the children involved in the study, 42.5% were female and 57.5% were male; both groups were the same in terms of gender. Results concluded that there was no difference between the two groups in terms of the gender composition of the samples (P>0.05) (Table 1). In addition, descriptive information such as economic situation, and education levels of the samples were shown in Table 1. Results have shown that there is a significant relationship between age and the individuals economic situation in both of the groups (P<0.001).
Study population characteristics.
| Group | |||||
|---|---|---|---|---|---|
| Index | Sub-index | Control (n=60) | Case (n=60) | Total | P-value |
| Age; mean±SD | – | 9.35±2.337 | 7.82±2.151 | – | <0.05 |
| Sex; n (%) | Male | 34 (56.6) | 35 (58.3) | 69 (57.5) | >0.05 |
| Female | 26 (43.3) | 25 (41.7) | 51 (42.5) | ||
| Economic situation; n (%) | Weak | 10 (16.7) | 13 (21.7) | 23 (19.2) | <0.05 |
| Intermediate | 38 (63.3) | 24 (40) | 62 (51.7) | ||
| Good | 12 (20) | 23 (38.3) | 35 (29.2) | ||
| Education (father); n (%) | Under Diploma | 8 (13.3) | 7 (11.7) | 15 (12.5) | >0.05 |
| Diploma | 14 (23.3) | 23 (38.3) | 37 (30.8) | ||
| Associate | 6 (10) | 0 (0) | 6 (5) | ||
| Bachelor | 19 (31.7) | 30 (50) | 49 (40.8) | ||
| Masters | 7 (11.7) | 0 (0) | 7 (5.7) | ||
| Phd | 6 (10) | 0 (0) | 6 (5) | ||
| Education (mother); n (%) | Under Diploma | 11 (18.3) | 5 (8.3) | 16 (13.3) | >0.05 |
| Diploma | 21 (35) | 21 (35) | 42 (35) | ||
| Associate | 9 (15) | 0 (0) | 9 (7.5) | ||
| Bachelor | 10 (16.7) | 34 (56.7) | 44 (36.7) | ||
| Masters | 9 (15) | 0 (0) | 9 (7.5) | ||
| Phd | 0 (0) | 0 (0) | |||
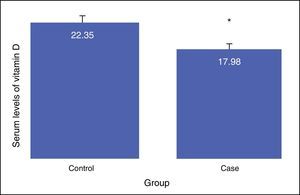
The results showed that there is a significant difference between the serum levels of vitamin D in both groups, with the mean serum level of vitamin D being lower in the case group (P<0.01). The mean serum levels of vitamin D of the subjects in the case and control groups were 17.98±8.68, and 22.35±6.26, respectively, as presented in Fig. 1. The severity of vitamin D deficiency in all subjects, 10%, 43.3%, 34.2%, and 12.5% were respectively severe, deficient, suboptimal, and optimal.
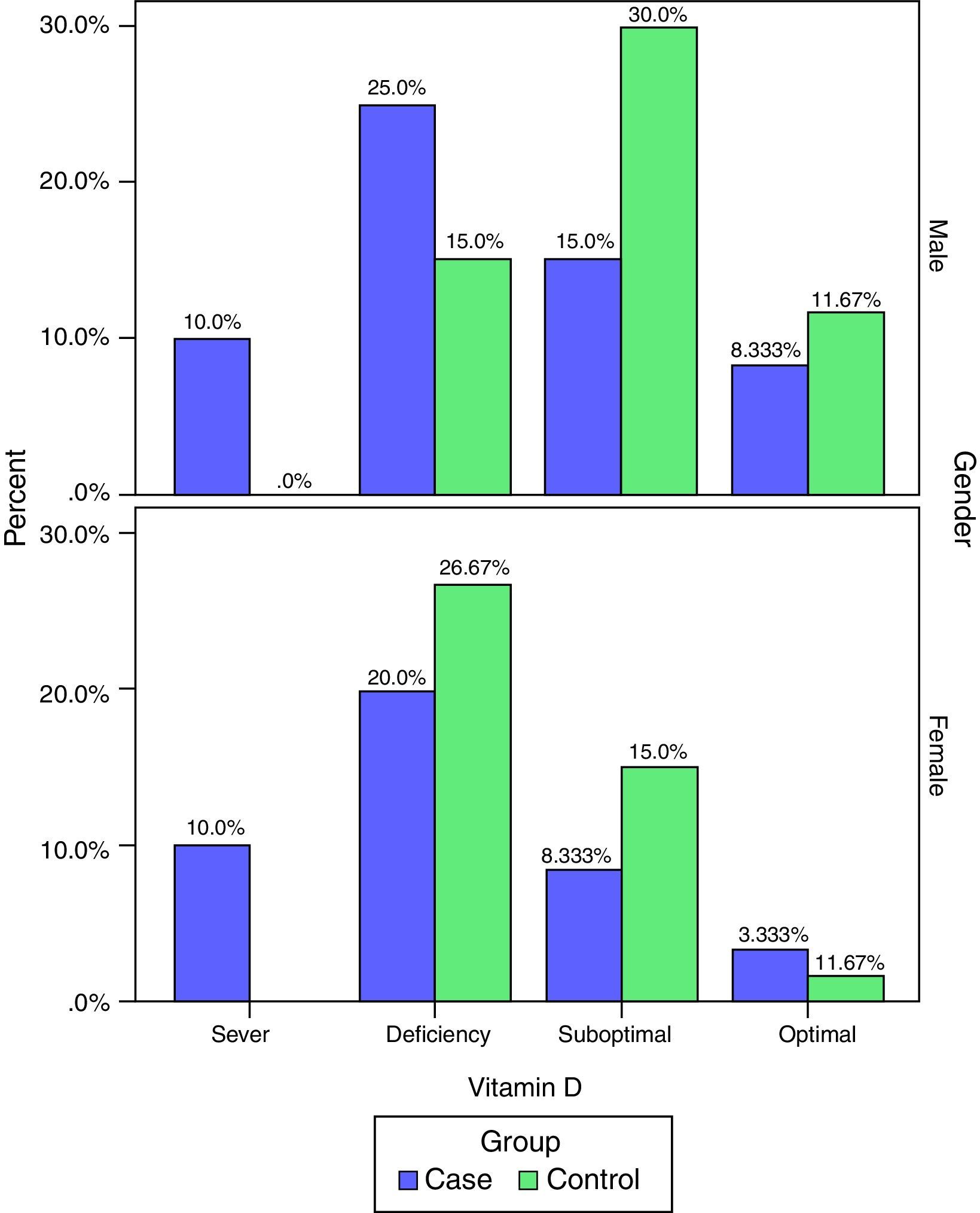
Vitamin D deficiency in the two groups (case and control), by separate levels (deficiency, suboptimal, optimal and sever) are presented in Table 2.
Comparison of the distribution of the severity of the vitamin D deficiency in the two groups.
| Variable | Status | Group | Mean±SD | Mann–Whitney U | P-value |
|---|---|---|---|---|---|
| Vitamin D | Deficiency | Case | 17.51±9.16 | 291.50 | 0.40 |
| Control | 16.40±2.63 | ||||
| Suboptimal | Case | 17.77±6.41 | 60 | <0.0001 | |
| Control | 24.9±3.18 | ||||
| Optimal | Case | 23.71±10.66 | 13 | 0.083 | |
| Control | 32.33±2.22 |
Due to the severe shortage only in the case group, comparing the mean at the severity level in case and control groups was not possible, but a significant difference in the mean suboptimal level of vitamin D were observed (P<0.0001). In other levels of vitamin D, significant differences were not reported (P>0.05).
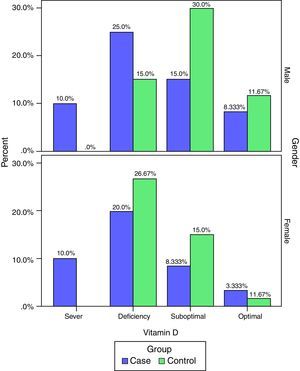
The frequency of vitamin D deficiency in both groups by term of gender are presented in Fig. 2. Based on Fig. 2, the frequency of severe shortage of vitamin D in the case group (male and female) have been reported at 10%, and also in the control group no cases of Vitamin D deficiency have been reported (0.0%). In addition, optimal vitamin D in male in both cases and the controls were more than vitamin D in female. But, based on the results of the chi-square test, there was no significant correlation between vitamin D and gender in children (P>0.05).
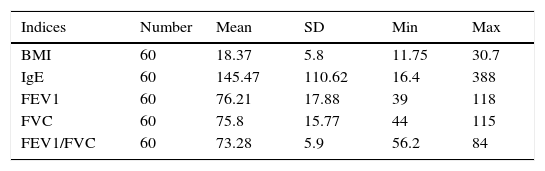
Mean of various indices associated with the severity of asthma in the case groupIn this study, the mean and standard deviation of FEV, BMI, IgE, FEV1, FVC, and FEV1/FVC were 76.21±17.88, 18.37± 5.8, 145.47± 110.62, 76.21±17.88, 75.8±15.77, and 73.28±5.9, respectively (Table 3).
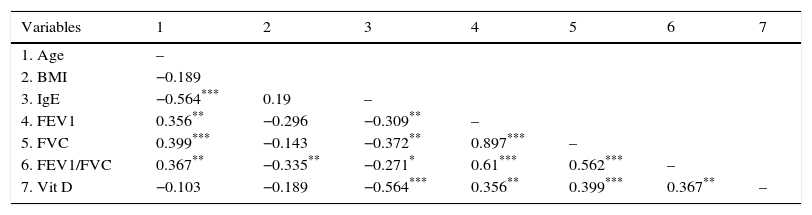
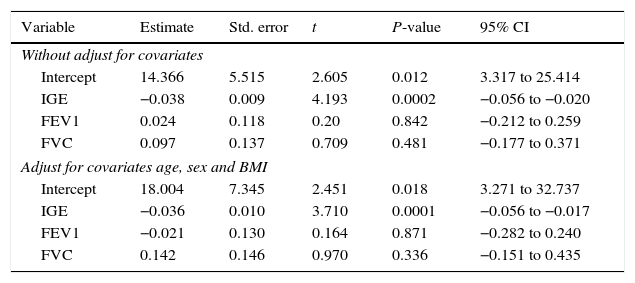
Correlation seen between vitamin D and other study variables in the case groupAccording to the results of the present study, there is a positive correlation between the levels of vitamin D with FEV1, FVC, and FEV1/FVC; furthermore, there is a negative correlation between the levels of vitamin D with IGE (Table 4).
Correlation between vitamin D and other study variables in the case group.
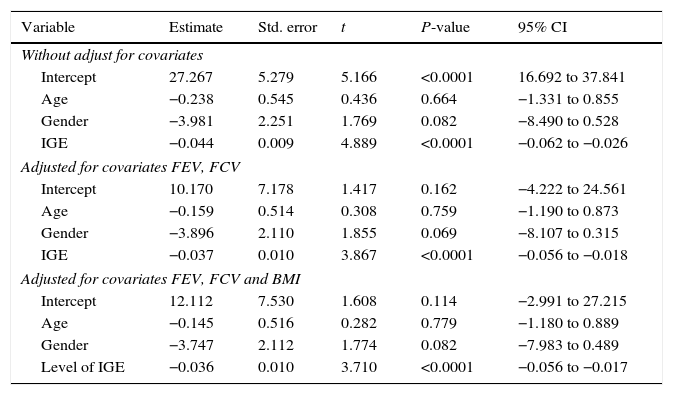
To investigate the relationship between vitamin D with the predictor variables: age, gender, and IgE, multiple regression models were used by controlling confounding variables: FEV, FCV, and BMI.
According to the results of the multiple regression model, there was no relationship between gender with vitamin D, but between the levels of vitamin D and IgE there was a significant negative relation. This relationship remained even after adjusting for FEV, FVC, and BMI.
The R-square for models 1, 2 and 3 was 0.341, 0.387 and 0.387 and the adjusted R-square was 0.305, 0.327 and 0.318 respectively. With respect to this information, Model 2 was the best model (Tables 5 and 6).
Association between serum levels of vitamin D with IgE, FEV1 and FVC in the case group, with and without adjusting for confounders (age, sex and BMI).
| Variable | Estimate | Std. error | t | P-value | 95% CI |
|---|---|---|---|---|---|
| Without adjust for covariates | |||||
| Intercept | 14.366 | 5.515 | 2.605 | 0.012 | 3.317 to 25.414 |
| IGE | −0.038 | 0.009 | 4.193 | 0.0002 | −0.056 to −0.020 |
| FEV1 | 0.024 | 0.118 | 0.20 | 0.842 | −0.212 to 0.259 |
| FVC | 0.097 | 0.137 | 0.709 | 0.481 | −0.177 to 0.371 |
| Adjust for covariates age, sex and BMI | |||||
| Intercept | 18.004 | 7.345 | 2.451 | 0.018 | 3.271 to 32.737 |
| IGE | −0.036 | 0.010 | 3.710 | 0.0001 | −0.056 to −0.017 |
| FEV1 | −0.021 | 0.130 | 0.164 | 0.871 | −0.282 to 0.240 |
| FVC | 0.142 | 0.146 | 0.970 | 0.336 | −0.151 to 0.435 |
Association between serum levels of vitamin D with age, gender and IGE in the case group, with and without adjustment for confounders (FEV, FCV and BMI).
| Variable | Estimate | Std. error | t | P-value | 95% CI |
|---|---|---|---|---|---|
| Without adjust for covariates | |||||
| Intercept | 27.267 | 5.279 | 5.166 | <0.0001 | 16.692 to 37.841 |
| Age | −0.238 | 0.545 | 0.436 | 0.664 | −1.331 to 0.855 |
| Gender | −3.981 | 2.251 | 1.769 | 0.082 | −8.490 to 0.528 |
| IGE | −0.044 | 0.009 | 4.889 | <0.0001 | −0.062 to −0.026 |
| Adjusted for covariates FEV, FCV | |||||
| Intercept | 10.170 | 7.178 | 1.417 | 0.162 | −4.222 to 24.561 |
| Age | −0.159 | 0.514 | 0.308 | 0.759 | −1.190 to 0.873 |
| Gender | −3.896 | 2.110 | 1.855 | 0.069 | −8.107 to 0.315 |
| IGE | −0.037 | 0.010 | 3.867 | <0.0001 | −0.056 to −0.018 |
| Adjusted for covariates FEV, FCV and BMI | |||||
| Intercept | 12.112 | 7.530 | 1.608 | 0.114 | −2.991 to 27.215 |
| Age | −0.145 | 0.516 | 0.282 | 0.779 | −1.180 to 0.889 |
| Gender | −3.747 | 2.112 | 1.774 | 0.082 | −7.983 to 0.489 |
| Level of IGE | −0.036 | 0.010 | 3.710 | <0.0001 | −0.056 to −0.017 |
The aim of the present study was to investigate the relationship between the serum levels of vitamin D with asthma and its symptom severity. In terms of gender, the number of people in both groups was the same. The results of this study showed that there is no significant difference between both groups in terms of the gender distribution. However, results showed that there was a significant relationship between the groups that were studied by age; this concludes that the mean age in the case group was less. The incidence rate of asthma is present at lower ages and is more, with increasing age its prevalence becomes less. Nearly 80% of asthma cases have been reported before the age of 6.19 The epidemiologic pattern of asthma over the last 30 years also confirms these findings. In a study by Rachel et al., the incidence of asthma in children aged 0–4 was 23.4/1000 was higher more than five times the same ratio in the subjects aged 12–17 (4.4/1000).21
In this study, the severity of vitamin D deficiency was also investigated. The results showed that the severity of vitamin D deficiency in all subjects had been 10% (severe), 43.3% (deficient), 34.2 (suboptimal), and 12.5% (normal). In addition, the results showed that the most frequent of the severe cases of decrease in vitamin and vitamin deficiency was related to the case group (asthma patients). Generally, vitamin D deficiency in the world is epidemically prevalent, and there is high prevalence even in areas with abundant sun exposure; by taking 25(OH)-D level of ≤20ng/ml as vitamin D deficiency, Gordon et al. reported a rate of 12.1% of this vitamin deficiency in American infants/toddlers.22 In another study in the United States, the rates of vitamin D deficiency and vitamin D insufficiency among people aged 0–21 were reported at 21% and 28%, respectively.23 In Tehran, capital of Iran, a study was conducted with the aim of examining the vitamin D deficiency in children 9–12 years old and the results showed that 72.4% studied children suffer from vitamin D deficiency.24 So, that research's outcomes are similar to our findings.
According to the findings of the present study, there is a significant difference between serum levels of vitamin D in the case (with asthma) and control groups, so that severe cases and vitamin D deficiency were related to the case group (with asthma). As noted, several studies have been conducted in this field, but their results were different. In a study, Uysalol et al. investigated the association between vitamin D deficiency and asthma in children 2–14 years old.23 The results indicated a significant difference in the level of vitamin D in the case and control groups, so that the mean and standard deviation of vitamin levels in the case and control were 16.6±8.5 and 28.2±19.5, respectively.23 Similar results have been seen in studies Hisham13 and Elnady15 in Egypt, Sharif25 and Alyasin14 in Iran, and Kolokotroni in Cyprus.26 But, there were different results observed in studies by Hughes, Devereux, and Hyppönen.27 In these studies, no relationship was seen between the levels of vitamin D with asthma. Here, to give an explanation for the inconsistency between these studies, we will discuss the aetiologies of the asthma.
The interaction between genetic factors and the environment of the people causing the inflammation in the air ducts. Studies clearly indicate the involvement of some genotypes in susceptibility to asthma and allergic problems, as well as the amount of responsiveness to asthma treatment.28,29 In addition, depending on the age range of the samples, the rate of response and the expression of asthma in different studies can be varied. Finally, some factors of variation in the results of the studies can be different allergens, such as foods; indoor allergens, including animal allergens, fungi, mites, … or outdoor seasonal allergens (pollen of trees, grass, etc.); cigarette smoke; cold air; chemicals; paints; ozone (O3); atmospheric changes in temperature and barometric pressure; air quality; exercise; heat and cold; and dehydration.30
As mentioned, the results of this study have clearly confirmed the relationship between vitamin D deficiency and asthma. In explaining the findings, the role of vitamin D in maintaining the homeostasis of safety and its effect on the innate and acquired immune system can be pointed out. It is known that vitamin D inhibits the proliferation and differentiation of T and B cells, and prevents the secretion of immunoglobulin.30,31 It also causes the induction of regulatory T cells and inhibits Th17 cells.32,33 Generally, this vitamin causes the secretion of anti-inflammatory cytokines such as IL-10 and inhibits inflammatory cytokines such as IL-1, IL-6,and TNF-α.34 On the other hand, this vitamin is able for induction of tolerogenic DC properties in DC cells through enzyme CYP27B1.35 Therefore, vitamin D shows a type of immunomodulatory and anti-inflammatory property.
In this regard, Agrawal showed that Vitamin D supplementation reduces inflammation of the air ducts and airway hyperresponsiveness in a murine model of allergic asthma. This study reported that vitamin D deficiency correlated with high BALF (bronchoalveolar lavage fluid) eosinophilia, increased pro-inflammatory cytokines, reduced BALF IL-10 levels, reduced blood T-regs, and increased expression of importin-α3 and Rel- A in the lung tissue.36 In another study, to evaluate the effect of vitamin D supplementation on eosinophilic and neutrophilic airway inflammation in patients with non-atopic asthma, de Groot et al. showed that Vitamin D reduces eosinophilic inflammation of the air ducts and can be useful in the treatment of asthma.37 In another study, Samaha et al. showed that vitamin D plays a role in airway remodelling.38 Probably, through increased responsiveness to corticosteroids, vitamin D reduces eosinophilic airway inflammation.39 In this field, various studies showed that vitamin D deficiency increases the risk of airway hyper-reactivity and reduces lung function.40
According to the findings of our study, there is a significant positive correlation between vitamin D deficiency and lung function tests such as FEV1, FVC, and FEV1/FVC. These observations are consistent with the results of the studies conducted by Alyasin14 and Elnadya.41 Also, Abd El Aaty showed a direct relationship between 1, 25 (OH) 2D, and lung function.13 Moreover, Jung demonstrated that a significant positive relationship exists between the level of 25-OH-functional lung VitD3 and lung tests of FEV1, FEV1/FVC.42 Samaha et al. showed that patients that are vitamin D deficient have an incomplete pulmonary function. In addition, increased FENO and higher numbers of exacerbations have been reported in these patients.38 However, Yoseph,43 Brehm,44 and Litonjua12 provided different results in their studies. The relationship between lung function with vitamin D has not been clearly specified yet. However, it is predicted that vitamin D improves lung function through the effect on the muscles, adjustment of the inflammation, and induction of anti-microbial peptides.45 It is known that vitamin D has a reverse relationship with circulating levels of Matrix Metalloproteinas (MMP-9). Matrix Metalloproteinas (MMPs) are enzymes that have a role in inflammation and cellular movement within the lung. It is known that these enzymes are involved in remodelling and inflammation process of COPD. Song et al. showed that pre-treatment of the human airway smooth muscle cells with 1, 25(OH)2D3 decreases production of MMP-9, disintegrin, and metalloprotease 33 (ADAM33) in the in vitro environment.45
In this study, a reverse relationship was observed between serum levels of IgE as an allergic indicator with vitamin D. This relationship remained even after the adjustment of FEV, FVC, and BMI. It seems that the relationship between vitamin D and allergic indicator such as IgE is independent from other effective factors such as FEV, FVC, and BMI.
In this regard, Brehm et al. investigated the relationship between vitamin D and the markers of severity of childhood asthma among 616 children in Costa Rica.44 In multivariate linear regression models, the results showed a reverse and significant relation between levels of vitamin D and IgE. These findings are consistent with the results of studies conducted by Uysalol46 and Hala,15 but in a study by Yoseph, no difference was seen between the levels of vitamin D with the serum level of IgE.43 In this study, 39 people were studied in two group cases and a control. Finally, a prescription of vitamin D for the case group did not cause a significant change in IgE, eosinophil count, high sensitivity C-reactive protein, FeNO levels, and inflammatory and anti-inflammatory cytokines (IL-4, IL-5, IL-10, and IL-17). Low size samples were one of the limitations of this study. Also, different results can be caused by a difference in choosing the type of samples, sample size, follow-up duration, seasons of review, and etc. Perhaps, through the inhibition of secretion of IL-4 IL-5, vitamin D inhibits too much increase in serum IgE levels; and in this way, it plays a role in controlling allergic diseases and asthma. However, this hypothesis requires more studies to confirm.
The results of this study suggest that serum levels of vitamin D in asthma patients have been less in healthy people, and also lung function has reduced in these patients. Of course, this point should be seriously considered that many factors play a role in asthma, so, more related variables should be considered in these patients. Also the serum levels of vitamin D in patients with asthma must be checked continuously.
Recommendations to researchers- 1.
To conduct interventional studies with larger sample sizes.
- 2.
To conduct studies in the various target groups and taking into account the different efficient factors.
- 3.
To conduct studies on the long interval and free or bioavailable levels of vitamin D.
- 4.
To perform the measurement of bone mineral density in patients with asthma.
- 5.
To minimise the amount of effective doses in the treatment of asthma (oral and systemic corticosteroids) with bone influences to control the disease with drugs with minimal side effects.
- 6.
To check the amount of food and medicinal effects on the disease and its severity.
- 7.
Measurement of vitamin levels after treatment with vitamin and its impact on asthma movement and the need for medications.
The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestThe authors declare that they have no conflict of interests.
This study was the result of a research project approved by the Research deputy of Kurdistan University of Medical Sciences and it was conducted under its financial support. We would like to appreciate those who paid special attention to this project.