Food allergies are inflammatory conditions mediated by Th2 and probably STAT-6 dependent immune responses.
Objective and designHere we investigated the role of Signal Transducer and Activator of Transcription 6 (STAT-6) in development of inflammation in peanut allergy.
MethodsTo induce food allergy, wild-type (WT) and mice deficient for STAT-6 (Stat6−/−) were sensitized with peanut proteins and challenged with peanut seeds.
ResultsWT animals lost weight and refused the peanut diet, in contrast to Stat6−/− mice, which had a better maintenance of body weight and more regular seeds’ consumption. The augmented peanut-specific IgG, IgG1 and IgE in the allergic WT was abolished in Stat6−/− animals that also presented increased IgG2a. There was an overall reduction in the gut mediators in the absence of STAT-6, including those related to inflammatory and Th2 responses, in contrast to a rising counter regulatory and Th1 reaction in Stat-6−/− mice. These animals had IFN-γ and IL-10 similar to WT after the four-week challenge. Most interestingly, Stat-6−/− mice had no intestinal damage, in contrast to WT animals, which had inflammatory infiltrate, tissue destruction, epithelial exulceration, edema, congestion and loss of villous architecture in the small gut segments.
ConclusionsSTAT-6 plays an important role in the establishment of the Th2 inflammatory responses and intestinal damage in peanut allergy.
The failure to establish or maintain gastrointestinal homeostasis leads to a breakdown of mucosal tolerance that may predispose to gut disorders, like food allergy.1 Adverse reactions to food antigens may develop rapidly and include cutaneous, respiratory or gastrointestinal manifestations, which may end in fatal anaphylactic reactions.2–4 The most common proteins able to induce such clinical reactions are those derived from cow's milk, wheat, egg, soy, shellfish, tree nuts and peanut,5 the latter being responsible for the majority of cases of food-induced anaphylaxis.6,7
The majority of food allergy cases are immunoglobulin E (IgE) mediated and this is not different in peanut allergy, since in susceptible individuals the peanut-specific IgE binds to its receptors on mast cells and basophils. The degranulation of mast cells induces the release of allergy mediators (such as histamine, prostaglandins and leukotrienes),8 which activate the production of chemokines and cytokines like IL-4 and IL-13 that in turn activate other inflammatory cells and generate the development of T helper 2 (Th2) immune response, which signals via STAT-6 activation.9,10 STAT-6 is a transcription factor that belongs to the Signal Transducer and Activator of Transcription family and is critical for a number of T lymphocytes responses, including the development of Th2 cells.11,12 STAT-6 deficient mice are not capable of performing IL-4-mediated functions including differentiation of Th2 lymphocytes, expression of cell surface markers and antibody class switching to IgE,11,13 most responses are also observed in vitro.11,13 Moreover, this transcription factor is mainly activated by IL-4 and IL-1314–16 and can enhance GATA-3, the main regulator of Th2 differentiation.17 Together, STAT-6 and GATA-3 lead to the augmented secretion of IL-4, IL-5 and IL-13 by the activated Th2 lymphocytes.18 Furthermore, mice lacking STAT-6 do not develop allergic diarrhea induced after repeated oral administration of ovalbumin,19–21 suggesting an essential role for this transcription factor in the allergy responses.
However, although it is clear that STAT-6 is involved in Th2 differentiation and effector functions, its role in the development of peanut allergic responses is still unclear, as are the immunological events associated to the intestinal damage that underlies the onset of food allergy. Accordingly, in the present work we used STAT-6 deficient mice and a murine model of peanut sensitization described previously by our group22 to elucidate the role of STAT-6 in the modulation of intestinal immunity during the development of food allergy in vivo.
Materials and methodsInduction of peanut allergyFor the development of food allergy, BALB/c wild-type (WT) and Stat-6−/− mice, from the same genetic background (6–8 weeks old, n=5animals/group), were sensitized with 100μg of peanut protein extract (PPE) in the presence of 1mg of aluminum hydroxide by subcutaneous injection. Three weeks later, another injection of PPE was performed in PBS, without alumen. After one week, challenge was initiated with peanut seeds for the next 30 days (four weeks) uninterruptedly, in the absence of the conventional chow (S+Peanut group).22 The experimental controls comprised the non-sensitized (NS) group, which received the first sensitization with PBS plus aluminum hydroxide, followed by a second injection with PBS only and was not submitted to the diet containing peanut seeds; the Peanut group, which was submitted to the same alumen or PBS injections as the NS group, but was fed peanut seeds for 30 days and the Sensitized group (S), which received the PPE twice (as S+Peanut group) but was not challenged with the peanut diet. Water was available ad libitum and mice weight was scored during all the experiment. For peanut ingestion, we quantified the total food consumption per group in a week and divided this value by the number of mice in the cage, which were five. The animal procedures were approved by the School of Medicine of Ribeirão Preto Institutional Animal Care and Use Committee (protocol number 017/2004).
Euthanasia and sample collectionMice were anesthetized, bled, and euthanized on day 30 after introduction of the peanut diet. Sera were stored for immunoglobulin detection and gut segments were collected for RNA extraction and histology procedures.
Histopathological analysisSmall gut segments (duodenum, jejunum, ileum and colon) were collected, fixed in 10% formaldehyde/PBS solution and embedded in paraffin. Sections (5μm) were stained with hematoxylin and eosin and evaluated for the presence of tissue inflammation.
Enzyme-linked immunosorbent assay (ELISA) for immunoglobulin detectionTo investigate the production of PPE-specific antibodies, the serum levels of IgG, IgG1, IgG2a and IgE were measured by ELISA on 96-well polystyrene ELISA plates (low binding, Corning; New York Costar, Acton, MA, USA) coated with PPE (20μg/well). All samples were analyzed twice, in 1:100 dilution and the results were showed as ELISA index, as previously described.23
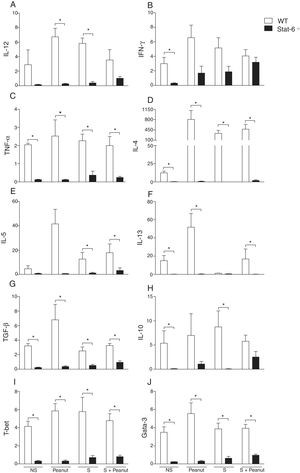
Real-time PCRThe extraction of total RNA from jejunum was performed using the TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) and Promega RNA extraction kit (Promega, Madison, WI, USA), according to the manufacturer's instructions. The complementary DNA (cDNA) was synthesized by a reverse transcription reaction (M-MLV reverse transcriptase, Promega) containing 1μg of RNA. Real-time PCRs were performed on the ABI Prism 7000 Sequence Detection System with specific primers (Table 1) and the SYBR-green quantification system (Applied Biosystems, Warrington, UK). The standard reaction conditions were: 95°C for 10min; 40 cycles consisting of 1min at 94°C, 56°C (1min) and 72°C (2min), followed by the standard denaturation curve. The results were depicted as mRNA expression of the NS, Peanut, S and S+Peanut animals, relative to naive mice, for both the WT and STAT6−/− groups. The relative level of gene expression was calculated according to the instructions from User's Bulletin (P/N 4303859) from Applied Biosystems, by reference to the β-actin in the sample, using the cycle threshold (Ct) method. Negative controls without RNA and without reverse transcriptase were also performed. Results showed one experiment representative of three.
Primers sequences used in real-time PCR assays.
| Primers | Sequences | |
|---|---|---|
| β-Actin | Sense | AGC TGC GTT TTA CAC CCT TT |
| Antisense | AAG CCA TGC CAA TGT TGT CT | |
| T-bet | Sense | CCC CTG TCC AGT CAG TAA CTT |
| Antisense | CTT CTC TGT TTG GCT GGG T | |
| GATA-3 | Sense | AGG AGT CTC CAA GTG TGC GAA |
| Antisense | TTG GAA TGC AGA CAC CAC CT | |
| IL-4 | Sense | CTG ACG GCA CAG AGC TAT TGA |
| Antisense | TAT GCG AAG CAC CTT GGA AGC | |
| IL-5 | Sense | GAG GTT ACA GAC ATG CAC CAT T |
| Antisense | TCA GTT GGT AAC ATG CAC AAA G | |
| IL-13 | Sense | ACC AAC ATC TCC AAT TGC AA |
| Antisense | ATG CAA TAT CCT CTG GGT CC | |
| TNF-α | Sense | TGT GCT CAG AGC TTT CAA CAA |
| Antisense | CTT GAT GGT GGT GCA TGA GA | |
| IL-12p40 | Sense | AGC ACC AGC TTC TTC ATC AGG |
| Antisense | GCG CTG GAT TCG AAC AAA G | |
| IFN-γ | Sense | GCA TCT TGG CTT TGC AGC T |
| Antisense | CCT TTT TCG CCT TGC TGT TG | |
| IL-10 | Sense | TGG ACA ACA TAC TGC TAA CCG |
| Antisense | GGA TCA TTT CCG ATA AGG CT | |
| TGF-β | Sense | GCT GAA CCA AGG AGA CGG AAT |
| Antisense | GCT GAT CCC GTT GAT TTC CA |
The significance level was fixed at 0.05. Tests of normality were applied and when data were considered normal, unpaired T tests or one-way ANOVA (followed by Tukey's test) were used. In case of non-Gaussian distribution, the Mann–Whitney or Kruskal–Wallis tests (followed by Dunn's) were applied. The graphs and statistical analyses were performed using GraphPad Prism 5.0 software.
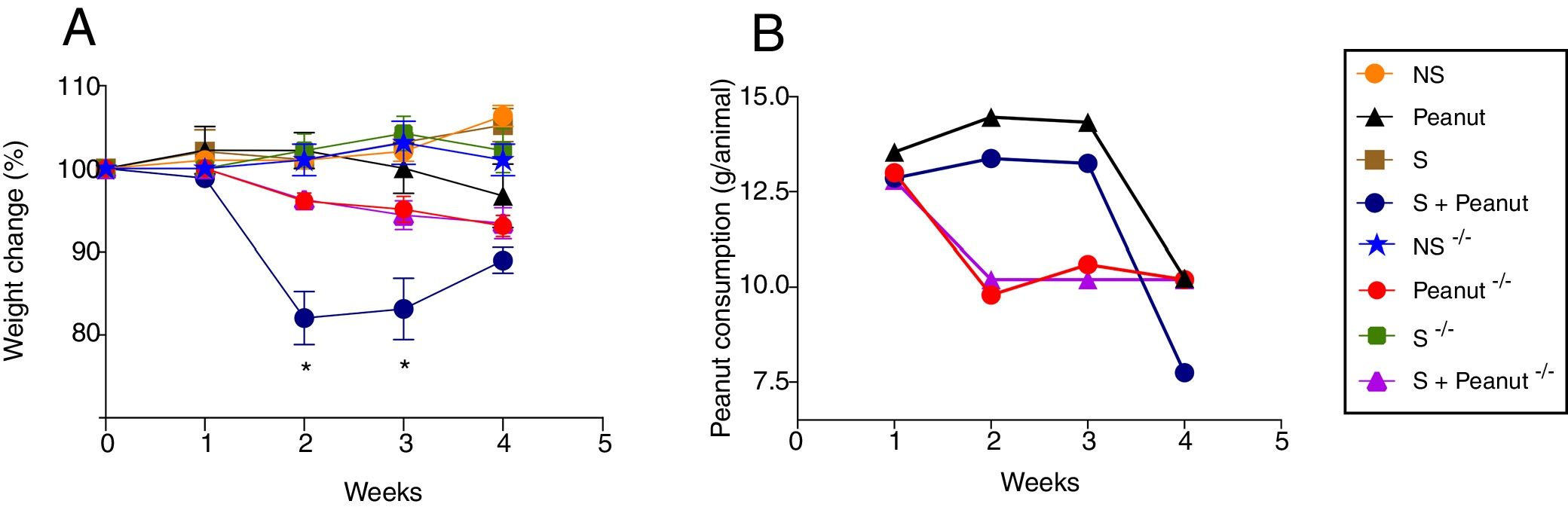
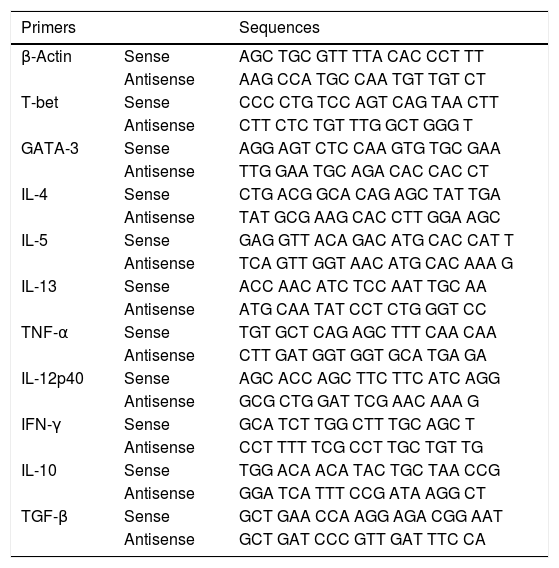
ResultsThe absence of STAT-6 rescues mice from weight loss induced by the food allergyFirst, to evaluate the importance of STAT-6 in the development of peanut allergy, mice were sensitized and challenged with peanut seeds, weighed weekly and followed for the development of diarrhea, bleeding in the feces or hypoactivity. The results showed that the main sign presented by WT animals submitted to peanut diet was loss of weight, especially during the second and third weeks after peanut challenge, in contrast to Stat-6−/− mice which had a significantly less prominent loss of weight in the same period evaluated (Fig. 1A). A decrease in peanut consumption in both the WT and the Stat-6−/− group accompanied the weight loss. WT mice began to refuse peanuts seeds around the third week of peanut challenge, while in the absence of STAT-6 there was an initial reduction in peanut ingestion followed by stabilization in the last three weeks of challenge (Fig. 1B). These data indicated that the transcription factor STAT-6, classically involved in the development of T-helper type 2 (Th2) cells, could play a protective role against peanut allergy.
Variation in body weight and peanut consumption in wild-type (WT) and Stat-6−/− post sensitization and challenge with peanut seeds. In (A), the gain or loss of weight after the four-week challenge is visualized as percentage of the weight variation related to the first day of peanut diet. (B) Peanut consumption throughout the chronic exposure to the seeds. Results are representative of four independent experiments. NS, non-sensitized mice; S, animals sensitized with peanut proteins extract (PPE); Peanut, mice non-sensitized with PPE but exposed to peanut seeds for four weeks; S+Peanut, animals sensitized with PPE followed by challenged with peanut seeds for four weeks. The symbol (−/−) represents the same experimental conditions for mice deficient in STAT6. *p<0.05.
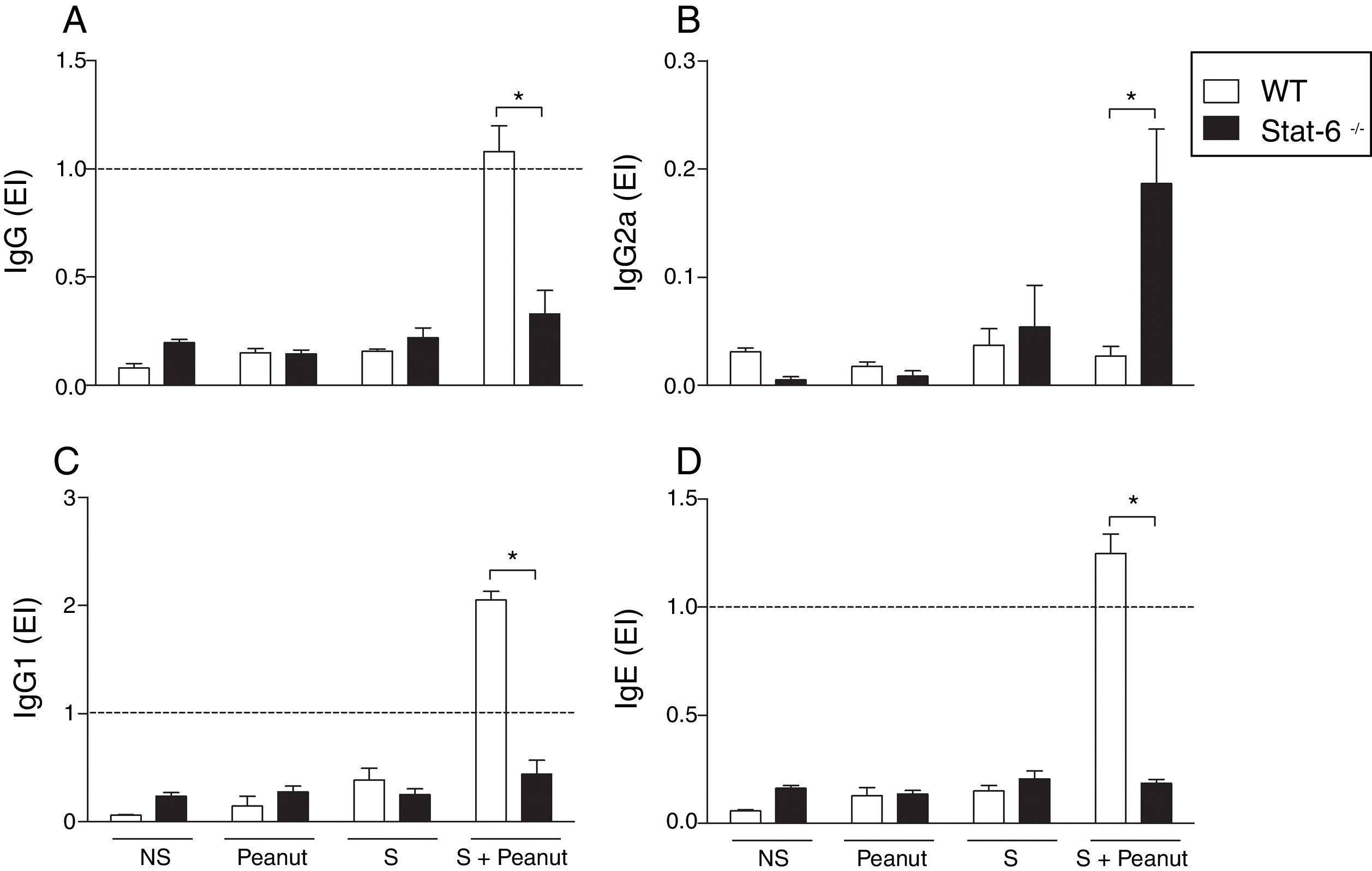
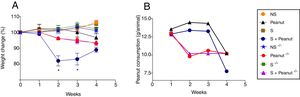
The production of PPE-specific antibodies in mice submitted to the protocol of development of food allergy was investigated in order to evaluate the role of STAT-6 in the humoral response during peanut allergy. The results demonstrated that in the absence of STAT-6, mice sensitized and challenged with peanut proteins (S+Peanut group) presented a significantly lower production of PPE-specific IgG, IgG1 and IgE antibodies, compared to the same experimental group, in the presence of STAT-6 (Fig. 2A, C and D, respectively). Quite the opposite, Stat-6−/− mice (S+Peanut group) exhibited a greater production of IgG2a antibodies by activated B cells, in comparison to allergic WT animals in the same conditions, as demonstrated in Fig. 2B, pointing to a shift toward a Th1 response in the absence of the transcription factor STAT-6.
Peanut-specific Th2 antibodies are abolished in mice deficient for STAT-6. WT and Stat-6−/− animals were sensitized with the peanut protein extract (PPE) and challenged with peanut seeds for four weeks (S+Peanut group), when sera were collected for quantification of peanut-specific antibodies, as described in Material and Methods. Results were expressed as ELISA index (EI), where values of EI >1.0 (horizontal dashed line) where considered positive. In (A) IgG, (B) IgG2a, (C) IgG1 and (D) IgE. Results are representative of four independent experiments. NS, non-sensitized mice; S, animals sensitized with PPE; Peanut, mice non-sensitized with PPE but exposed to peanut seeds for four weeks. *p<0.05.
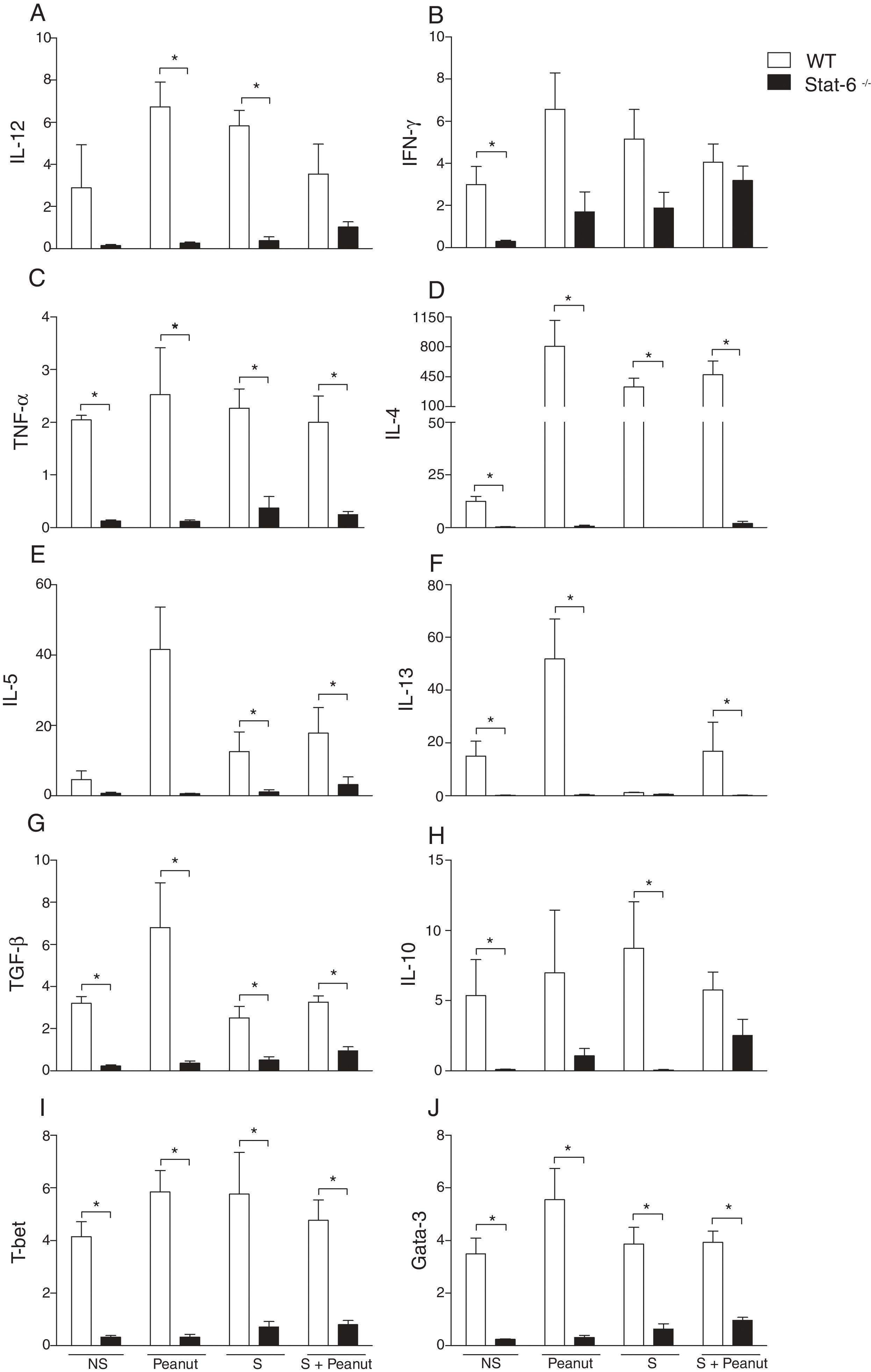
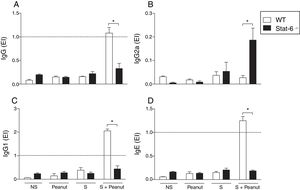
To define the role of the Th2/STAT-6-dependent responses in the modulation of gut mucosal immunity in peanut allergy, we characterized the expression of Th1, Th2 and regulatory cytokines beyond the transcription factors T-bet and Gata-3 in the jejunum of WT and Stat-6−/− animals. The expression of IL-12p40 was increased in the WT group exposed to peanut seeds or sensitized to PPE in comparison to the animals submitted to the same experimental protocol, in the absence of STAT-6 (Fig. 3A) Similar results were observed in TNF-α, IL-4, IL-5, IL-13, TGF-β, T-bet and Gata-3 mRNA expression, which was overall reduced in Stat-6−/− mice compared to WT (Fig. 3C, D, E, F, G, I and J, respectively). Nevertheless, although the expression of IFN-γ and IL-10 was higher in WT NS or S groups, there was a notable increase on these cytokine messages in Stat-6−/− mice sensitized and exposed to peanut seeds, indicating that a Th1 or a counter regulatory response is raised in the gut of these animals during induction of food allergy. In fact, after 30 days of challenge with peanut seeds, both the WT and Stat-6−/− sensitized mice produced equal amounts of IFN-γ and IL-10 mRNA (Fig. 3B, respectively). These results demonstrated the relevant role for STAT-6 in the induction of Th2 pathological responses in the gut and in the modulation of cytokine expression or regulatory factors in this model of food allergy.
STAT-6 modulates gut cytokines and the expression of Th1/Th2 transcription factors in response to peanut exposure. (A) IL-12, (B) IFN-γ, (C) TNF-α, (D) IL-4, (E) IL-5, (F) IL-13, (G) TGF-β, (H) IL-10, (I) T-bet, (J) Gata-3 mRNA were quantified by real-time PCR in jejunum segments collected after four weeks of peanut challenge. Results of WT or Stat-6−/− mice were demonstrated as mRNA expression relative to the mRNA detection in their naive counterparts. The relative levels of gene expression were calculated by reference to the β-actin in each sample using the cycle threshold method and were expressed as arbitrary units, according to the Applied Biosystems User's Bulletin #2 (P/N 4303859). Results are representative of three independent experiments. NS, non-sensitized mice; S, animals sensitized with peanut proteins extract (PPE); Peanut, mice non-sensitized with PPE but exposed to peanut seeds for four weeks; S+Peanut, animals sensitized with PPE followed by challenged with peanut seeds for four weeks. *p<0.05.
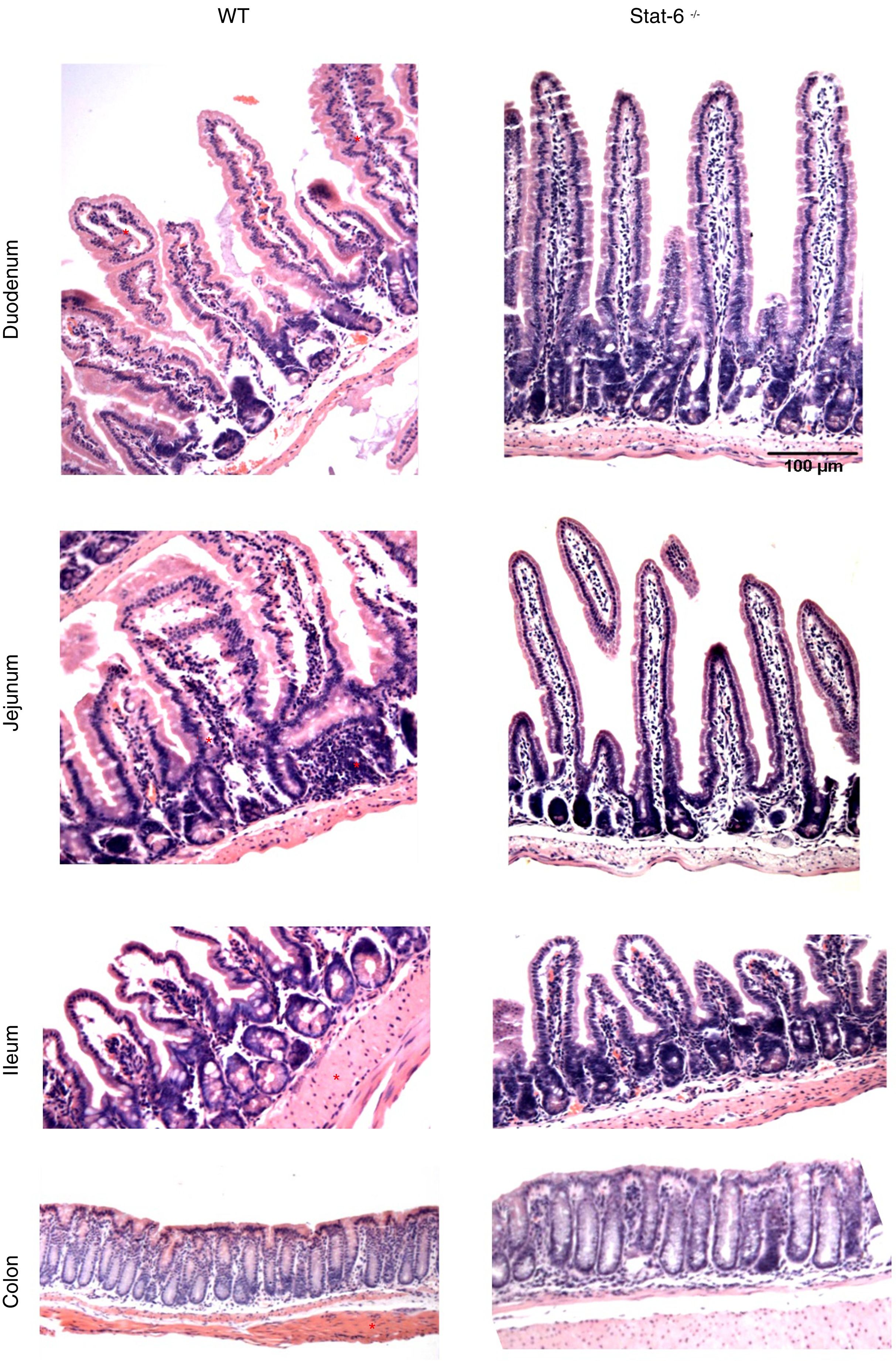
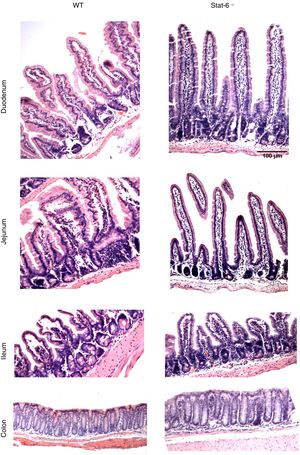
The induction of peanut allergy in BALB/c mice led to histopathological alterations in the intestinal tissues, especial in the small gut. There were augmented signs of inflammation in mice sensitized and exposed to peanut seeds, as visualized by a more intense accumulation of leukocytes, edema and congestion in lamina propria from duodenum, jejunum and ileum of WT animals (Fig. 4, left panel). These findings were markedly prominent in the jejunum segments, which presented loss of villous architecture, epithelial exulceration and a remarkable inflammatory process, in comparison to the same segment of Stat-6−/− mice. Indeed, in the absence of this transcription factor, there was no apparent damage in the small gut tissues, except for some minor signs of edema and congestion in the ileum (Fig. 4, right panel). Furthermore, as expected, no alterations were observed in the colon of WT or Stat-6−/− mice (Fig. 4, lower panel). Altogether, these results suggested that STAT-6 might influence the intestinal injuries caused by repeated allergen exposure in mice sensitized to the development of food allergy.
Histopathological analysis reveals intestinal damage in wild-type (WT) but not in Stat-6−/− mice sensitized and challenged with peanut seeds. Animals were sensitized with peanut proteins extract (PPE) and challenged with a chronic exposure to peanut seeds for four weeks (S+Peanut group), when the gut samples were collected for paraffin processing and histology evaluation. Note the inflammatory infiltrate, tissue destruction, epithelial exulceration, edema, congestion and loss of villous architecture in the duodenum, jejunum and ileum samples of WT mice (*). These alterations were almost absent in Stat-6−/− mice and in the control groups of both lineages (NS, S and Peanut groups, data not shown). The colon samples did not depict any sign of inflammatory destruction too. Results are representative of three independent experiments. Scale bar=100μm.
Polymorphisms in STAT-6 were already associated to allergy in the Japanese population,24 nut allergy in the UK25 and food sensitization in children from Mexico.26 A recent study investigated two single nucleotide polymorphisms (SNPs) in STAT-6 gene and showed that the variants were associated to food allergy, since the clinical presentation of peanut allergy symptoms and the higher specific IgE were related to the identification of the A alleles gene variants.27 Then, here we used mice deficient for STAT-6 to describe the importance of this molecule in peanut allergy.
The STAT-6 signaling pathway is central to the development of allergic diseases, because of the induction of IL-4/IL4R-dependent Th2 reactions that drive the local and systemic manifestations of allergy. After cellular activation, STAT-6 is phosphorylated and translocate to the nucleus, where it regulates the expression of genes associated to the development of allergic responses, such as GATA-3, which potentiates the expression of IL-4, IL-5 and IL-13 that in turn stimulates Th2 differentiation and activation of cells involved in allergic reactions. Consequently, there is class switching to IgE production that mediates mast cell degranulation and allergic inflammation.28 Upon exposure to food antigens, the Th2 inflammatory response develops with the induction of systemic and mainly gastrointestinal damage.29,30
Our first data pointed to a control of weight loss in the absence of STAT-6. Indeed, in previous studies of our group we characterized this model of peanut allergy and showed that weight loss is one of the signs of the allergic disease in mice.29 These alterations could be related to intestinal inflammation or refusal of the seeds, which represented the chronic exposure of the allergen by oral route. Similarly, IL-4−/− mice, submitted to the same protocol of peanut allergy induction, gained weight23 and Stat-6−/− mice did not present such a notable reduction in peanut consumption as the WT group, reinforcing the relevance of the Th2 response to the disease establishment. We also described the breakdown of intestinal tolerance by food antigens, the importance of the immune regulation29 and the involvement of B cells in the inflammation caused by food allergy.22 So, the local inflammation and tissue damage induced by repeated exposure to peanut seeds in mice sensitized to peanut proteins were dependent on an imbalance in regulatory responses, together with a skewed Th2 profile and an intense influx of plasma cells in the gut lamina propria. These leukocytes were also involved in the production of allergen specific IgE and IgG1 antibodies.22,23,29
The presence of specific Th2 immunoglobulins, such as IgE, is one of the hallmarks of food allergy and may be correlated to increased risk of severe allergic manifestations such as anaphylaxis because of antibody-dependent mast cell degranulation.31 Here, the remarkable reduction of allergenic antibodies in the absence of STAT-6 clearly showed the involvement of this transcription factor in the humoral pathogenic response of food allergy, thus pointing to this molecule as an important future target for immunotherapy approaches, such as the blockage of IgE antibodies32 or cytokines in allergic patients.33
The STAT-6/Th2 axis is also relevant to the gastrointestinal inflammation caused by food allergy, since STAT-6 mediates the accumulation of eosinophils in eosinophilic esophagitis34 and participates in the induction of eotaxin-3,35 a chemokine associated to the recruitment of eosinophils to inflamed sites. In the present work, we showed an important modulation of cytokines production in the gut during peanut allergy onset in WT mice and the ablation of most of them in Stat-6−/− animals. However, an outstanding opposite response mediated by IL-10 and IFN-γ seemed to rise in these knockout mice, probably in an attempt to balance the local deregulated immunity established by the exposure to the allergenic antigen in WT group. In accordance, a contrasting Th1 response is observed in non-allergic individuals36 and the production of IL-10 is one of the most important tolerance mechanisms in the gastrointestinal tract.37 Indeed, the generation and function of regulatory T cells specific to allergens is impaired in mice with augmented susceptibility to food allergy, which presents a Th2 reprogramming dependent on STAT-6.38
Corroborating our results, another study of mice sensitization to food antigens showed that STAT-6 is fundamental to the development of allergic diarrhea, as a consequence of ovalbumin administration,19 thus pointing to an intestinal damage caused by the allergen. Therefore, in line with the previous studies of the group,22,23,29 it is evident that the chronic exposure to allergenic food in the gut, especially in the jejunum, not only led to a Th2 deleterious inflammatory response, but also to a disruption of the organ architecture. These alterations were clearly abolished in Stat-6−/− mice, thus confirming the role of this molecule in mediating the immune response related to villous destruction and lamina propria inflammation in peanut allergy. These local reactions probably involved leukocytes such as eosinophils and mast cells, besides their mediators. Hence, the cytokine IL-4 and the subsequent signaling dependent on STAT-6 are fundamental to the expansion of mast cells in food allergy.39 Mucosal mast cells participate in the gut inflammation induced by food allergens in susceptible hosts, especially by the production of IL-9, IL-13 and proteases after antigen challenge. The development of this specific population of mast cells (MMC9) is fundamental to the susceptibility of food allergy and depends on T cells, IL-4 and the transcription factor STAT-6.40
Finally, the etiology of allergy is multifactorial and may be underlined by a genetic basis along with environmental factors and a deregulated immune response, that together culminate in exacerbated reactions to ingested foods. Therefore, here we showed that the transcription factor STAT-6 plays an important role in determining the Th2 responses and intestinal damage after peanut sensitization, thus pointing to this molecule as a relevant target for future studies aimed at developing novel therapies for food allergies.
Compliance with ethical standardsAll applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
FundingThis study was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq grants no. 310174/2016-3; 311882/2013-7; 307915/2009-3) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grant no. 03/03809-3).
Conflict of interestThe authors declare that they have no conflict of interest.
We are grateful to Vivinai Nardini for helping with figures preparation. This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).