Renal allograft anastomotic pseudoaneurysms (AP) are rare though associated with high rates of graft loss and mortality. Intrinsic transplantation mechanisms regarding active immunosuppression and/or chronic rejection increase susceptibility for their development, having a prognostic impact. We present a case report of successful surgical treatment of a 6cm right iliac transplant patch pseudoaneurysm, diagnosed following an episode of acute obstructive renal insufficiency caused by AP ureteral compression. Treatment consisted, by a transperitoneal approach, in partial AP resection, antegrade termino-terminal renal artery re-implantation in the external iliac artery and right lower limb revascularization with extra-anatomical femoro-femoral crossover 8mm PTFE bypass. There were no post-operative complications and renal function normalized to previous values.
Os falsos aneurismas anastomóticos (FA) de transplantes renais são raros. O seu tratamento está associado a elevadas taxas de perda do enxerto e mortalidade. Mecanismos intrínsecos à transplantação como a imunosupressão e/ou rejeição crónica aumentam a susceptibilidade para o seu desenvolvimento, tendo também impacto prognóstico. Apresentamos um caso clínico de tratamento cirúrgico de FA do patch de enxerto renal com 6cm, diagnosticado na sequência de episódio de insuficiência renal aguda obstrutiva, condicionada por compressão ureteral pelo FA. O tratamento consistiu, por abordagem mediana transperitoneal, em ressecção parcial do falso aneurisma com re-implantação da artéria renal na artéria ilíaca externa topo-a-topo em posição anterógrada e revascularização do membro inferior direito através de bypass extra-anatómico femoro-femoral cruzado com prótese PTFE 8mm. Não ocorreram complicações pós-operatórias e a função renal normalizou para valores basais.
Anastomotic pseudoaneurysms (AP) associated with renal transplants are relatively rare with an incidence <1%,1 being however associated with high rates of complications such as allograft loss, infection, rupture and even death. There are few cases described in the literature. Treatment challenge is to perform AP exclusion, preserving the renal graft, avoiding injuries to adjacent structures or repercussions for lower limb arterial and venous circulations.
Case report48 year-old male patient, history of chronic renal insufficiency due to chronic glomerulonephritis, hypertension, dyslipidaemia and erectile dysfunction. After an initial period of renal substitution therapy by peritoneal dialysis submitted to renal transplant in right iliac fossa, in August 2005. Kidney donor was a cadaver. Acute tubular necrosis was registered after graft implantation. Patient was immunosuppressed with tacrolimus, mycophenolate mofetil and prednisone, developing chronic graft dysfunction [basal creatinine (Cr) 1.5mg/dl].
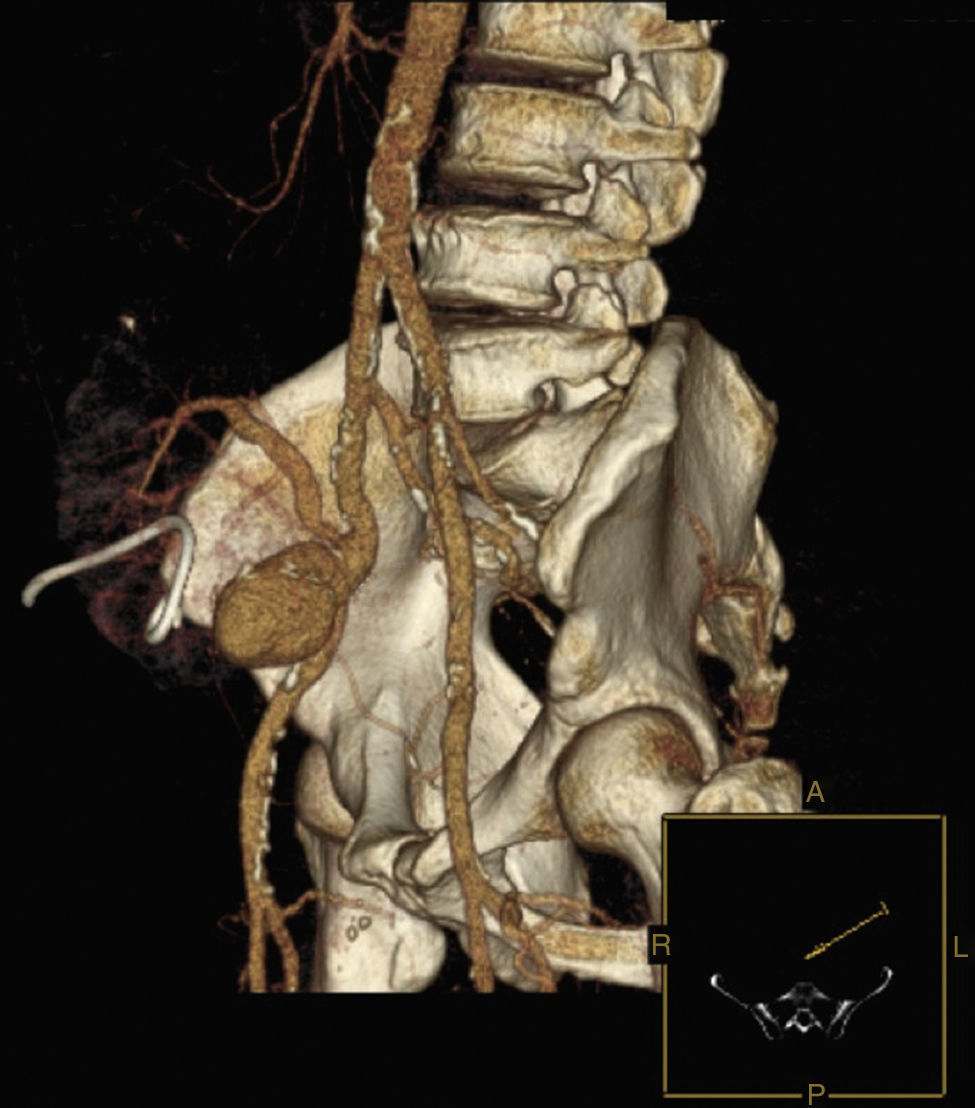
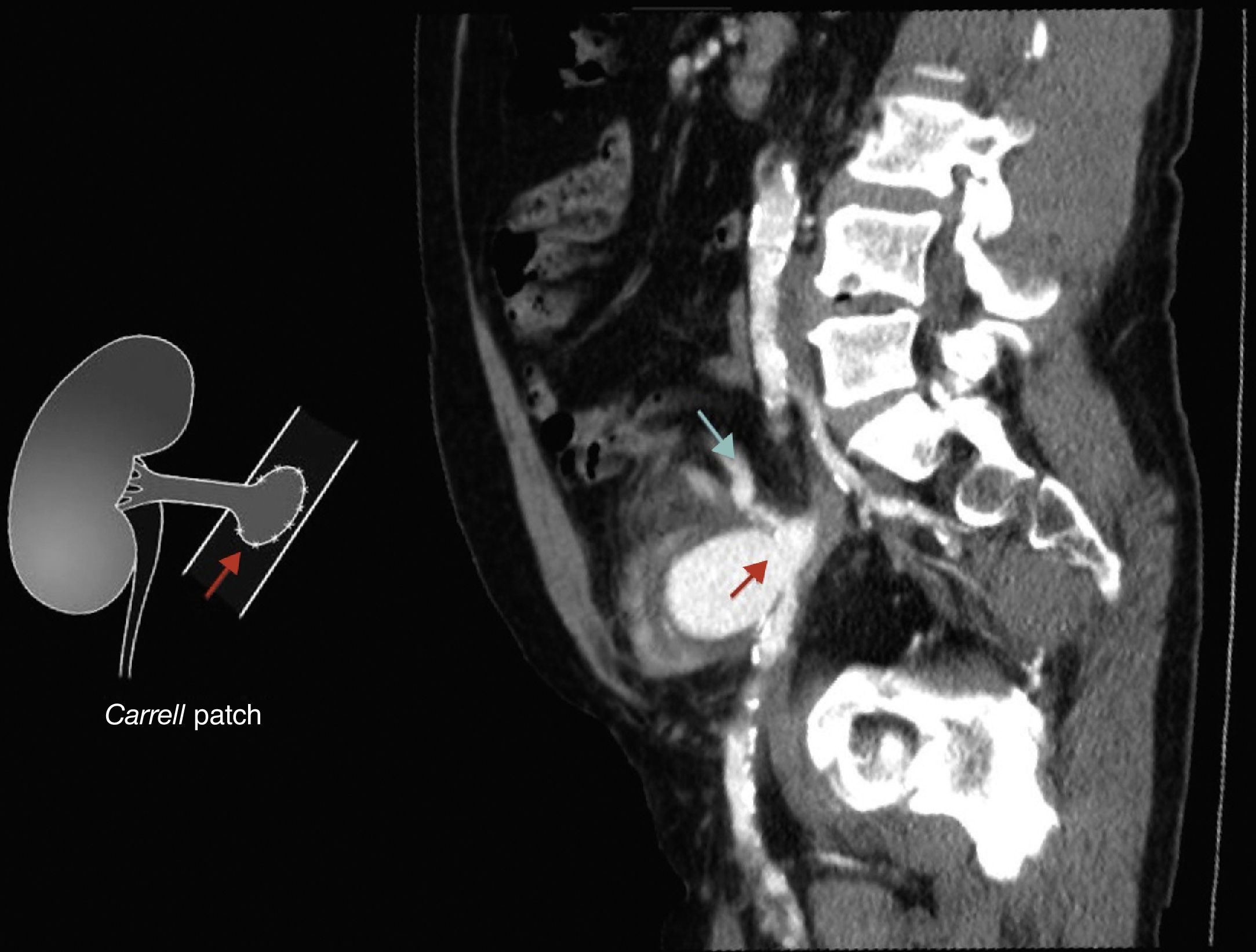
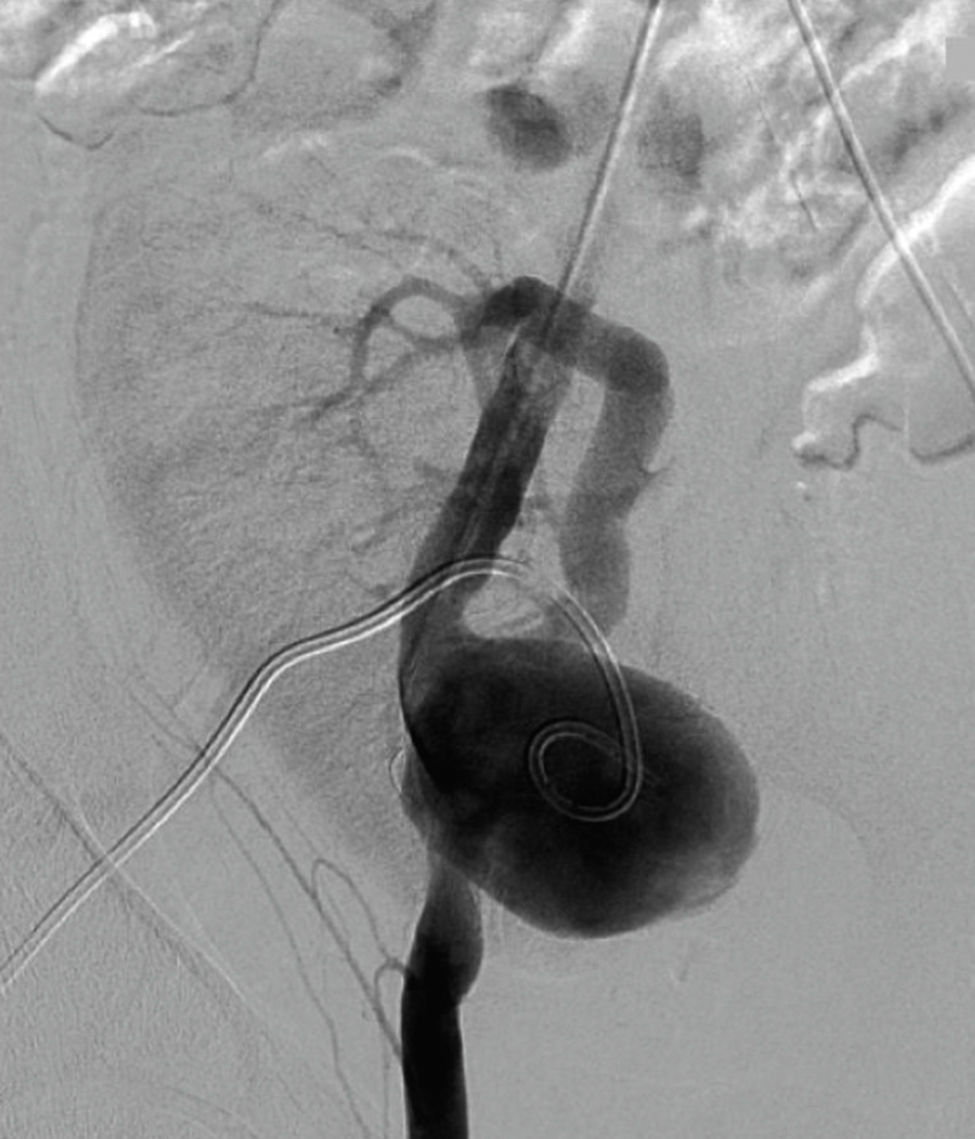
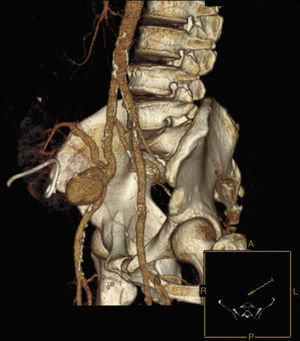
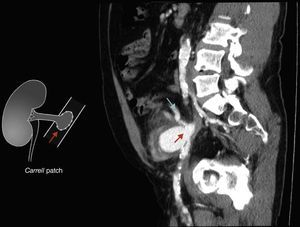
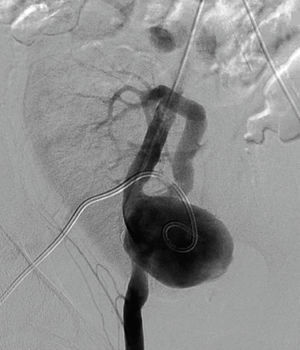
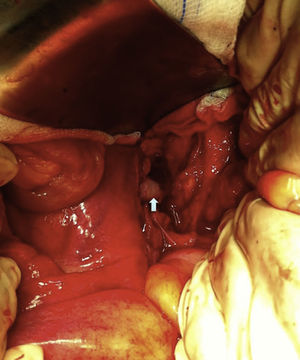
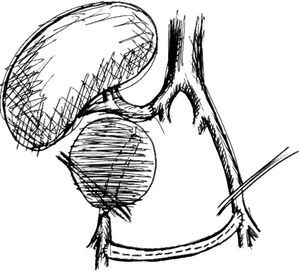
Six years after transplant patient presented acute worsening of renal insufficiency (Cr 3mg/dl), oliguria, without symptoms of graft pain. Etiological investigation concluded the origin was obstructive, post-renal, with the presence of significant uretero-hydronephrosis (renal pelvis diameter of 4cm). Eco-guided percutaneous urinary derivation nephrostomy was performed, not improving renal function. Repeated ultrassonographic kidney analysis revealed focal dilation of graft artery. CT-angio diagnosed a 6cm anastomotic pseudoaneurysm (AP) of renal artery implantation patch (Carrel patch), without evidence of rupture (Figs. 1 and 2) but leading to compression of the ureteral system. It was afterwards performed an angiographic study to obtain a better characterization of AP morphology and to plan intervention (Fig. 3).
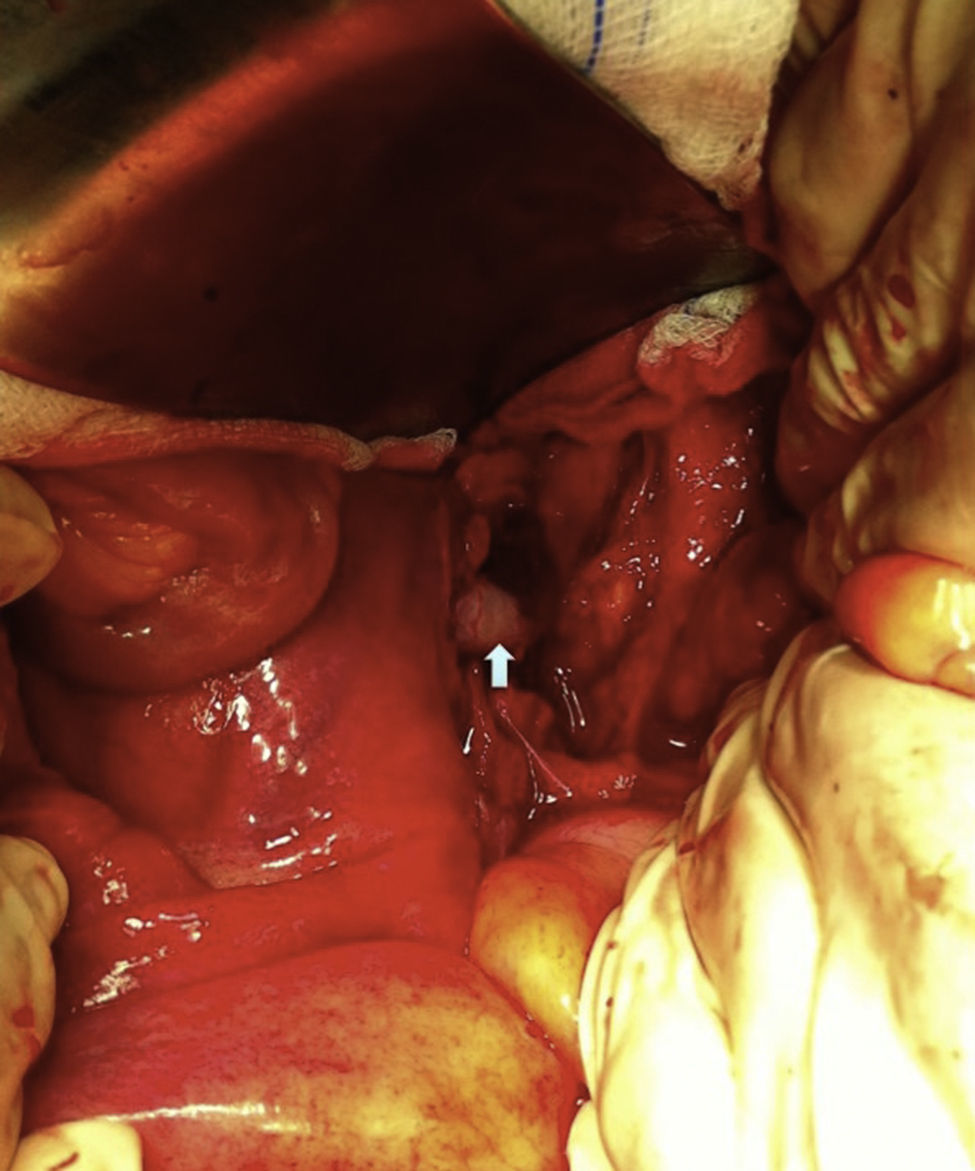
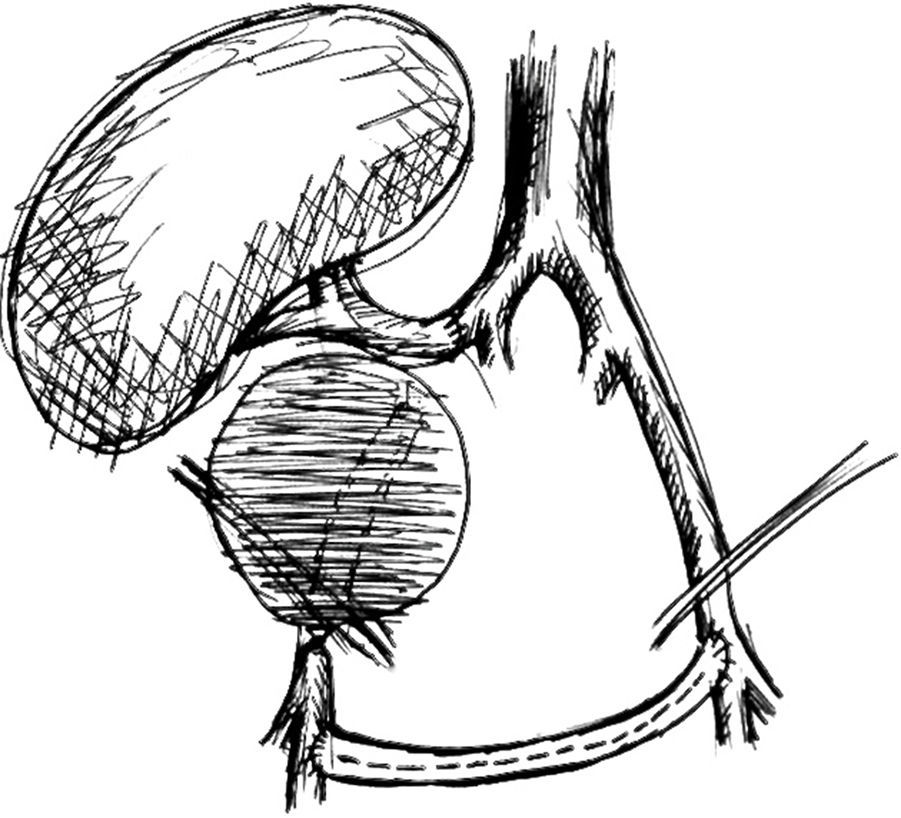
Surgical treatment consisted, by a median laparotomy transperitoneal approach, in a termino-terminal antegrade re-implantation of the renal artery on the external iliac artery (time of renal warm ischaemia <10min) (Fig. 4), followed by partial aneurysm resection and right lower limb revascularization with extra-anatomical femoro-femoral left>right crossover 8mm PTFE bypass (Fig. 5). Extensive retroperitoneal fibrosis was encountered which made the dissection of renal vessels particularly difficult. Kidney perfusion solutions were not used since it was performed a single rapid anastomosis to perfuse the kidney as first stage of the procedure.
Post-operative period was uneventful and Cr rapidly lowered to basal levels. Patient had an isolated fever peak on the 3rd post-operative day with analytic elevation of inflammatory parameters, reason for which was stared empiric antibiotherapy (amoxicillin/clavulanate). Hemocultures and aneurysm thrombus were negative for infection. Nephrostomy and algaliation were removed on the 9th post-operative day after a pyelography confirmed inexistence of extrinsic ureteral compression.
The patient is being followed-up for 4 years, with a stable renal function (Cr 1.3mg/dl), patent crossover bypass with bilateral palpable normal pedal pulses.
DiscussionDue to the relative low incidence of AP in renal allografts (small series or isolated cases reported in literature) the occurrence and management of this pathology are still subject of some debate.1–3 Aetiology of renal allograft AP's can be multifactorial: suture line defects and anastomotic dehiscence secondary to infection are well known causes. It is also speculated on the role of arterial wall degeneration secondary to immunologic reactivity phenomena similar to those involved in chronic allograft rejection.4,5 Although intra-renal aneurysms are demonstrably associated with these phenomena, currently there is not clear evidence to prove a direct association with extra-renal aneurysms.2 On the other hand, in a published series of combined renal allograft and post-transplantectomy patch anastomotic pseudoaneurysms, tissue immunohistochemical analysis showed findings consistent with chronic rejection with diffuse T CD4+lymphocyte infiltrate at the donor renal artery (non-self) in 45% of patients.2 Either way, immunosuppression status plays an important role in this pathology.
As far as clinical presentation is concerned, AP are usually asymptomatic and diagnosed incidentally, but can present with symptoms of fever and anaemia, compression of adjacent structures, renal graft dysfunction or bleeding/shock due to spontaneous rupture. Small asymptomatic AP in the absence of infection can be managed conservatively and monitored with regular follow-up.1 Formal indications for treatment are the presence of symptoms, rapid growth and diameter >2.5cm, which in practice corresponds to almost every cases at the time of diagnosis.4
Main treatment options include surgery1,2,4 or endovascular treatment.2,3,5–7 Conventional surgery is “gold-standard” for suspected cases of infectious aetiology. In non-infected AP it also offers several alternatives for arterial reconstruction thus improving the possibilities for graft preservation: renal artery can be re-implanted in the hypogastric, common or external iliac arteries (directly or with an associated interposition bypass) or in a latero-terminal fashion on a bypass for lower limb revascularization. Nevertheless, in practice, allograft preservation rates are quite low. It is important to point out, however, that in a significant number of these reported cases, at presentation there was a severe allograft dysfunction and/or infection and the best treatment ultimately consisted in a transplantectomy. After AP surgical excision, arterial reconstruction is mandatory to prevent lower extremity ischaemia: options include anatomic revascularization with an interposition bypass or in cases of suspected infection with an extra-anatomical femoro-femoral crossover bypass (silver coated or antibiotic impregnated). These prosthetic bypasses, when not associated with significant lower limb arterial disease, have acceptable medium and long-term patency rates.
There are recent isolated published cases of endovascular treatment with covered stents with Chimney6 and Periscope7 techniques with successful preservation of renal graft, however they are only possible when there is a very favourable iliac anatomy and non-existence of infection. Endovascular treatment has its major importance and applicability in emergent rupture situations to control active bleeding in unstable patients as a bridge to surgery. There is also a report of hybrid treatment in which allograft artery was re-implanted in the common iliac artery and AP excluded with a covered stent.8 Isolated cases also describe echo-guided injection of thrombin in smaller AP but with an important potential thromboembolic risk.9
Regarding prognosis, rates of renal graft loss, morbidity and mortality are very high, especially in infectious and ruptured cases.2
In our case, although there was no evidence of infection, a bypass in an anatomical position was not feasible due to the extensive retroperitoneal fibrosis encountered, which precluded a safe ipsilateral retroperitoneal tunnelling with significant risk of lesion to renal hilum structures. For this reason, we decided to re-implant the renal artery directly in the external iliac artery that was immediately adjacent, with only one anastomosis necessary to reperfuse the kidney and as the first step of the procedure, which allowed a shorter time of renal ischaemia, absence of post-operative acute tubular necrosis and graft preservation. In this case common and external iliac arteries were concentrically calcified, did not have a favourable anatomy for an exclusive endovascular exclusion (Fig. 2) and most importantly endovascular treatment would not resolve immediately the ureteral compression.
ConclusionsTreatment of renal transplant AP is associated with high rates of graft loss. Intrinsic transplantation mechanisms regarding active immunosuppression and/or chronic rejection increase susceptibility for their development and have prognostic impact, requiring further scientific research. Open surgery in our opinion is “gold-standard” for AP treatment in infectious situations and as well as in elective cases as it allows several possibilities for arterial reconstruction and graft preservation, moreover if some kind of decompression is mandatory. Endovascular treatment should be first considered in cases of rupture with haemodynamic instability or in non-infected high-risk surgical patients with favourable anatomy. Infectious complications, the main responsible for the morbidity/mortality, should be aggressively prevented and treated.
Conflicts of interestThe authors have no conflicts of interest to declare.