Dame Sheila Sherlock
After reading this review, the reader will have become aware of the following:
- 1.
Those parameters defining an adverse reaction to drugs in general and hepatic damage caused by the same in particular;
- 2.
Those quantitative methods used for evaluating the problem of hepatic toxicity caused by drugs from society’s point of view;
- 3.
Understanding the main characteristics of those mechanisms facilitating generating hepatic damage by drugs; and
- 4.
Critically analysing those actions, which as doctors we must implement in handling a patient suffering from drug-induced hepatic toxicity.
The authors have followed the following methodology in preparing this structured systematic review:
- 1.
A question has been posed basically focused on the clinical problem raised by the issue at hand;
- 2.
A coherent search strategy has been followed for finding and reviewing the pertinent literature published on the topic,
- 3.
A clear system has been used for selecting those published articles which could be useful for the review;
- 4.
A rigorous analysis has been made of those articles reviewed; and
- 5.
5. Inferences regarding handling have been drawn following those guidelines proposed by Evidence-based Medicine, based on reviewing the articles and the information obtained from them, from the most credible to the least solid, from the methodological point of view.
What are the mechanisms, consequences and action which should be executed in patients having an adverse hepatic reaction to drugs?
Search strategyAn electronic search was made using the MEDLINE. The MESH terms investigated were, “Adverse drug reaction” or “Drug-induced Liver Disease” or “Hepatotoxicity” or “Drug induced Hepatic injury” and “Pathogenesis.” Random experimental efficacy and safety studies and welldesigned Cohort and Case and Control analytical studies were included as well descriptive studies such as technical reviews, updates, narrative reviews, chapters in books, series of cases and case reports. No limitations were placed on the language and the review was done on articles published between January 1990 and January 2003.
System used for selecting articlesNeither published abstracts nor literature considered to be in the grey area have been included in this review. When characterising the topic, the authors estimated that both those random studies traditionally considered to be the most solid for establishing causality and case reports could provide useful information for this structured review. Studies of different compound and drug toxicity mechanisms used with experimental animals were also systematically analyzed.
Analysing the quality of those articles reviewedThe index proposed by Jadad et al was used for estimating the random studies’ validity, confidence and pertinence.1 Those recommendations made by Sacket et al2 for evaluating studies regarding risk were followed for determining the same parameters in analytical studies. Case series and case reports were interpreted in the author’s criteria as to whether they were relevant or not for review purposes.
Content, guidelines and recommendationsFinally, some guidelines for understanding and managing patients having an adverse hepatic reaction to drugs are proposed based on the exhaustive, systematic and structured review of the topic, these in turn being based on scientific evidence having the greatest credibility, validity, trustworthiness and pertinence.
Definitions “or the art of having the point of departure clear”An Adverse Reaction to Drugs has been defined for the purpose of this review as that proposed by Edwards and Aronson.3 This describes, “A type of detected dangerous or non-placentera reaction, resulting from an intervention related to the use of medicinal products predicting risks for their future administration, meriting their prevention or a specific treatment or an alteration of the dose or even the definitive suspension of using the product.” It should be emphasised that the term, “Adverse Effect” is preferable to other terms such as “Toxic Effect” or “Collateral Effect”. A toxic effect is that which is present as the consequence of an exaggeration of a desired therapeutic effect that is not common at normal dose. For example, if one gives 80 to 120 mgs of furosemide per day to a cirrhotic patient having ascitis, then such patient will present dehydration; this is a toxic effect.
It cannot be too clearly stated that a toxic effect is always related to the ingested dose. On the other hand, a collateral effect occurs by another mechanism and could be related to the ingested dose. For example, an anti-cholinergic effect related to a tricyclic anti-depressive dose is an adverse effect, since its action is not associated with the therapeutic purpose, even though such adverse effect could prove to be useful (i.e. it can simultaneously become a beneficial adverse effect in treating some manifestations of Irritable Bowel Syndrome).
An anaphylactic allergic reaction to a particular drug is another adverse effect but in this case it is not related to the dose used. In an attempt to make the definitions even more specific, the terms “Adverse effect” and “Adverse reaction” can be used interchangeably. What really differentiates them is the point of view from which they are evaluated; the adverse effect represents that point of view of a particular drug generating it and the adverse reaction deals with the point of view of the patient to whom it is happening. However, these two must be differentiated from the “Adverse event” representing whatever type of adverse happening which a patient presents whilst taking the drug and could necessarily be imputed to taking it.3
Adverse reactions to drugs can be classified into six types4:
- 1.
Those related to the given dose. These are characterized by being frequent, related to a drug’s pharmacologicallogical action, are predictable and are associated with low mortality. An example of this type of toxic adverse reaction can be seen at central nervous system level in some patients with hepatic encephalopathy treated with metronidazol. Another example of this type of non-toxic but collateral reaction is asthenia or lack of motor capacity developed by some patients with portal hypertension receiving treatment with beta-blockers. The management of this type of adverse reactions is based on straightforward strategies such as reducing the dose or considering the additive effects of some other intervention or co-therapy.
- 2.
Those not related to the given dose. This type of drug reaction is characterized by being infrequent, not related to a drug’s pharmacological action, unpredictable and having a poor outcome. These types of adverse reactions consist of immunological ones presented in hepatitis caused by halotane and idiosyncratic ones presented in hepatitis caused by sulphas. The treatment of this type of adverse reaction is difficult; sometimes inmunosuppresser-based therapy works, but it is most important to suspend using the drug immediately and avoid its being prescribed again during the patient’s lifetime.
- 3.
Those related to the dose and time. This type of adverse reaction is rare and related to accumulation of the drug. For insistence, patients with autoimmune hepatitis who have been receiving steroid treatment for a long time present a clinical picture of the hypothalamic-pituitary becoming suppressed. The usual management of this type or adverse reaction includes decreasing the dose or gradually suspending it.
- 4.
Those reactions associated with the time when a drug is taken. This type of reaction is rare; it is usually associated with the prescribed dose and is manifested or becomes apparent some time after a particular drug has been used. An example of this type is the development of cancer in patients who have used azathioprine for the treatment of autoimmune hepatitis. No treatment has been discovered to date for this type of adverse reaction.
- 5.
Those related to suspending taking a drug. This type of adverse reaction appears rapidly on suspending treatment; they are rare too. An example of this type of adverse reaction is when variceal haemorrhage develops in patients who have been receiving beta-blocker treatment for portal hypertension and which was then suspended for whatever reason. The treatment is to begin administering that particular drug again.
- 6.
Those related to an unexpected failure in treatment. This type of adverse reaction is very common. It is related to an inappropriate dose being prescribed, or because of interaction with other types of drug. An example of this type of reaction is poor control of chronic hepatic encephalopathy in patients being treated with lactulose and an antibiotic selectively eliminating the bacteria able to split the lactulose molecule, as happens in around 20% of those patients receiving lactulose concomitantly with metronidazole. The treatment of this type of adverse reaction is based on changing the dose or suspending the concomitant drug which is causing the therapeutic interference.
Talking directly about the liver, the following terms can be defined. Hepatotoxicity is the general term referring to that damage suffered by the liver caused by taking drugs or other chemical compounds.5 An adverse hepatic reaction to drugs is represented by unintentional, damaging effects incurred by the prescribed dose commonly used for use in prophylactic or therapeutic treatment treating.5 These nosological entities are difficult to define because the biochemical liver function test is used for determining them, but this bank of tests could show some alterations only as a reflection of the liver’s adaptive response to the drug and not necessarily indicating liver damage. To date, any elevation in hepatic enzyme level greater than three or more times the normal range has been considered to represent liver damage.
Clinical and histological expression of this damage in turn obeys a wide spectrum, varying from very slight alteration in the hepatic profile having minimum symptoms to developing an important clinical picture of necro-inflammatory disease, fulminant hepatic failure, cirrhosis or even hepatic cancer. It is considered that when establishing a diagnosis of drug-induced hepatic disease, then the nature of the damage hepatic must be confirmed from the histological point of view.5
Concerning the problem or its importance: focusing on the real impactChemical agents which can produce an adverse reaction having the liver as their target can be found in nature (usually called “natural drugs” by patients, as they protect them in a certain way with an “aura of benevolence”) or they can be chemical or pharmaceutical industry sub-products.
The problem and its importance must be appreciated from two points of view; the first represents real pathology from society’s point of view (with all its costs and consequences) and the second represents the frequency and risks for the community. From the first point of view, we can state that the adverse hepatic reactions to drugs are responsible for around 40% to 50% of the cases of hepatic diseases attended by hepatologists,6 approximately 5% to 10% of the cases of severe or icteric hepatitis meriting being hospitalized are the consequence of an adverse reaction to drugs. They are also responsible of 40% of the cases of severe hepatitis in people older than 50.7 In some series of articles, this type of reaction is responsible for around 25% to 30% of the cases of fulminant hepatitis.8 Half of all cases of acute hepatic failure in the USA are the consequence of an adverse drugs reaction.9 We estimate that 1,000 drugs have been involved in causing hepatic damage on more than one occasion, 100 causing acute hepatic failure and 400 causing some type of change of biochemical hepatic test.9
From the second point of view, suitably designed epidemiological studies confirm the rarity with which drugs being commonly used in clinical practice are associated with adverse hepatic reactions. This is a consequence of the tight supervision and scientific rigor imposed by international bio-security agencies on all medications during design phases I and II for evaluating security and later in pharmacy-supervision in clinical use (post marketing studies). This process provides an opportunity for detecting many cases of hepatic toxicity and has been why medication having an excellent theoretical basis in terms of efficacy has not been able to be used in a clinical setting. An outstanding example of this type of adverse reaction is what happened with troglitazone which, in spite of having demonstrated its enormous therapeutical potential in treating diabetes type 2 in the first phases of its development, had to be withdrawn from the market due to the cases of fulminant hepatic failure which it caused.
In any event, and as a consequence of this monitoring of the drugs currently being used, it can be said that those which are accepted for clinical use are very safe and have little capacity for damaging the liver. For example, the risk of hepatic damage due to non-steroid anti-inflammatory drugs being prescribed is only 1-10 out of each 100,000 individuals exposed.10 Also, only 1-5 out of each million people ingesting a combination of clavulanic acid and amoxycillin develop hepatitis.11
Some aspects employed by clinical epidemiology for measuring the frequency with which diseases are presented should now be defined for a little more scientific treatment of these diametrically opposite points of view. The most suitable way of estimating the RISK generated by a particular drug causing an adverse reaction at hepatic level is by measuring Accumulated Incidence.12 This is obtained by dividing the number of cases developing an adverse hepatic reaction during the follow-up time by the number of subjects free from whatever type of hepatic alteration at the time when they began to be exposed to a drug from the beginning of the follow-up.
Accumulated incidence = # new cases/# subjects exposed free from disease at the beginning of being exposed to a particular drug.
Technically speaking, accumulated incidence is not equivalent to the individual risk to which a person is exposed to a specific adverse outcome. It is more a way of estimating individual risk calculated from the general population by taking a sample of the exposed population. The rate of reported cases regarding adverse reaction to drugs is an inaccurate indicator for communicating the risk of such reaction occurring, because this depends on the doctor’s awareness in recognizing the case and the motivation forreporting it. It is obvious that these processes could be highly susceptible to great imprecision. To avoid such problems and to be able to have more exact indicators for estimating the real risk of developing hepatotoxicity due to a particular drug, the most suitable epidemiological methods include Adverse Event Monitoring guided by prescription and Case and Control studies. The latter have been particularly useful in defining Attributable Risk or the Etiological Fraction representing the proportion of cases occurring from a determined outcome attributable to an exposition of interest. Two of the clearest examples of the above are those studies relating the contribution of consuming aspirin with developing Reye’s syndrome and developing hepatic tumours from consuming oral contraceptive pills.
It should be stated that in the great majority of hepatic damage related to consuming medicaments, drugs are the only cause of such damage. In other cases, the drugs used can be facilitating damage, increasing risk, even though damage can be presented in their absence. In other words, this type of drug is not enough or necessary to produce damage. Examples of these types of “dangerous friendships” are contraceptives with developing Budd-Chiari Syndrome and methotrexate with developing fibrosis in patients having fatty liver.
Symbology: or the strategy of following cluesThe behaviour of adverse reactions at hepatic level induced by drugs follows some symbolic signs which are quite characteristic for each type of drug and which it can be said become their “digital fingerprints.” They are expressed in three ways:13 from the point of view of their latency, their clinical-histological manifestations and their genetic expression. These characteristics obviously allow causality to become established in many cases, orientating treatment and, in other cases, they serve to state association. These elements become the doctor’s early recognition signs, providing the opportunity for that early, opportune, specific treatment necessary for improving prognosis.
From the latency point of view, there are drugs whose genuine characteristic is to be intrinsically toxic and taken in large doses such as acetaminophen, cocaine and iron salts developing hepatic damage in a very short time (24-72 hours). These are usually accompanied by renal damage. Some other types of medication develop hepatic damage in periods of intermediate latency (1-8 weeks). This type of reaction is characteristic of idiosyncratic hypersensibility as occurs with phenitoin and sulphas. These reactions are frequently preceded or accompanied by clinical and cutaneous manifestations of hypersensibility. Finally, other drugs have periods of retarded latency even up to one month following suspending medication, as happens with clavulanic acid/amoxycillin mixture. Other medications induce damage following being ingested for months or years, as happens with isoniazide, troglitazone or methotrexate.
Rucam score
| Item | Description | Score |
|---|---|---|
| 1. Time to onset | Before starting drug or more than 15 days after stopping drug – incompatible unrelated From the beginning of drug 5 ~ 90 days Suggestive +2 < 5 or < 90 days Compatible +1 From cessation of drug = 15 days +1 | +2 |
| 2. Course | After cessation drug, ALT decrease: = 50% within 8 days – Highly suggestive +3 = 50% within 30 days – Suggestive +2 = 50% after 30 days. Inconclusive 0 < 50% after 30 days – Against –2 | |
| 3. Risk factors | Etanol: Presence +1 Absence 0 Age: = 55 years +1 = 55 years 0 | +1 |
| 4. Concomitant drugs | Time to onset incompatible 0 Time to onset compatible but unknown reaction –1 Time to onset compatible with known reaction –2 Role proved in this case –3 | 0 |
| 5. Non-drug causes | Gr I (6 causes): HAV, HBV, HCV, biliary obs, alcoholism, acute Hypotension Gr II CMV, EBV, HSV, Other underlying disease Rule out Gr I & Gr II +2 Rule out Gr I +1 Rule out 5 or 4 of Gr I 0 Rule out < 4 of Gr I –2 Probable –3 | 0 |
| 6. Previous information of drug | Reaction unknown 0 Reaction published but + Reaction labelled in the product’s characteristics +2 | +2 |
| 7. Rechallenge | Doubling of ALT with single drug: Positive +3 Doubling of ALT with drugs: compatible +1 Increased of ALT but < normal: Negative -2 or plasma concentration as toxic +3 or validated lab. Test: Possible +3 Negative –3 | 0 |
| Total score: 7 | = 0: excluded | |
| 1~2: unlikely | ||
| 3~5: possible | ||
| 6~8: probable | ||
| < 8: highly probable |
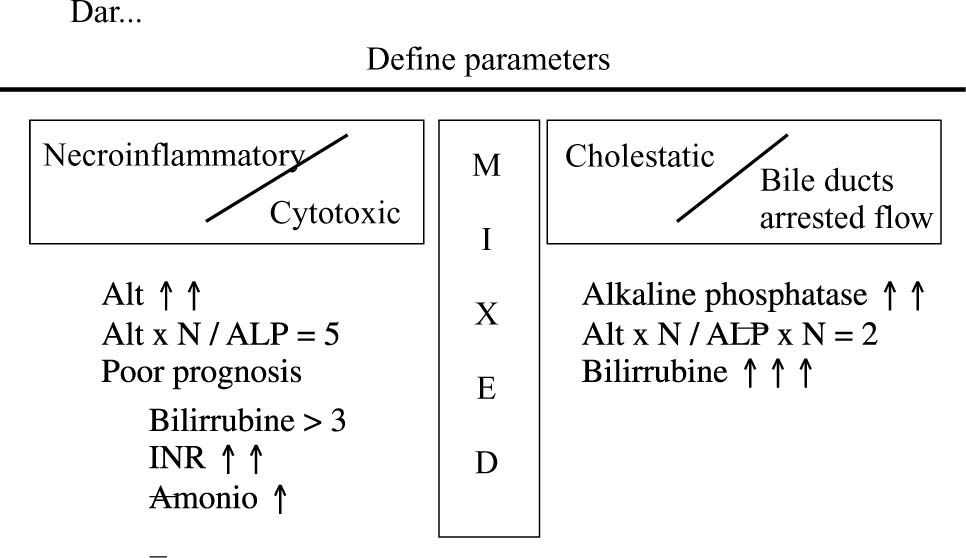
From the clinical-histological expression point of view, these could correspond to the following morphological characteristics, with their respective examples (Figure 1).
Acute hepatitis/necroinflation. This type of reaction is mainly produced by acetaminophen, cocaine, Ecstaxis, isoniazide and ketoconazole.
Chronic hepatitis/necroinflamation. This type of reaction is mainly produced by nitrofurantoine, methyldopa, dantrolene and minocyclin.
Cholestasis/acute necroinflamation. This type of reaction is mainly produced by erythromycin, sulindac, clorpromacin, phenitoin and sulfonamides.
Chronic cholestasis. This type of reaction is mainly produced by transmethyl, anabolizing hormones and contraceptives.
Miscellaneous. Some drugs produce characteristic damage with clinical expressions related to the anatomical site where damage was induced. Examples of these would be: methotrexate and developing fibrosis; amiodarone or tamoxifen and developing steatohepatitis; chemotherapy and developing veno-oclusive disease; valproic acid and fatty degeneration of small vacuoles; steroids and developing fatty liver of the large, intermediate and small vacuole.
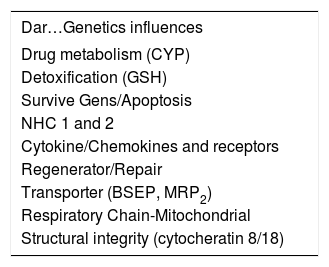
The last aspect or point of view to be taken into account and which participates in constructing the “digital fingerprint” for drug-induced hepatic damage is the Genetic Menu (Table I). In our criteria, this aspect has become the element having most transcendence in the near future, since its enormous utility has established it in the position of predicting or anticipating hepatic damage, making it without doubt the most significant strategy for preventing damage and that preceding hepatic damage caused by many drugs.
Genetic influences altering physiological and/or metabolic path of drugs which might be associated with developing toxicity.
| Dar…Genetics influences |
|---|
| Drug metabolism (CYP) |
| Detoxification (GSH) |
| Survive Gens/Apoptosis |
| NHC 1 and 2 |
| Cytokine/Chemokines and receptors |
| Regenerator/Repair |
| Transporter (BSEP, MRP2) |
| Respiratory Chain-Mitochondrial |
| Structural integrity (cytocheratin 8/18) |
Following the discovery of the human genome, we have learned several not just curious but rather enormously useful things. We now know that on average two randomly chosen unrelated people share 99.9% of their DNA sequences.14 However, given that the human genome has 3 billion base pairs, two humans having no type of family relationship have around 3 million base pair variations in their genes.
It is well-known that patients respond very differently to the same medication; it is estimated that genetic variations are responsible for 20% to 95% of a drug’s variable disposition, efficacy and eventual toxicity.15 Variations in the sequences of those genes responsible for encoding the enzymes entrusted with metabolism (between 50 and 100 enzymes participating in drug metabolism are subject to genetic polymorphism, variations in Cytochrome 450 being the most important), transport and affinity for membrane and intracellular-transporter receptors explains these differences. Such fingerprints are indelible, unmodifiable, but essentially predictable. There is no doubt that in the future it will be routine clinical practice to request a patient’s genetic panel allowing a doctor to orientate formulation (type, dose, mode of being applied, duration, etc) before prescribing medication for a patient, according to the findings regarding disposition, efficacy and potential toxicity given by ordering their particular genetic code.
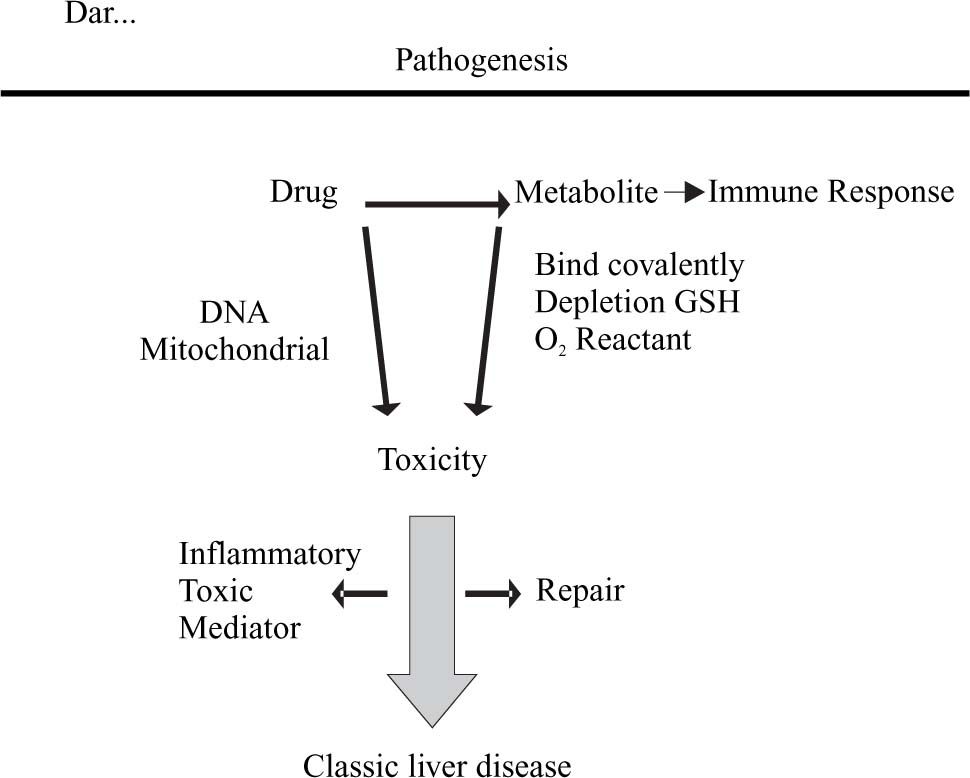
Pathogenesis: “Modulating the corruption”One of the most dramatic advances in the field of adverse hepatic reactions to drugs has been in knowing about those processes leading to establishing hepatic damage. It can be stated that the majority of drugs in themselves are not lethal; the great percentage of adverse damage is mediated by their metabolites and, as an important portion of drug metabolites are generated through Cytochrome P450 action, this is why genetic variations in this Cytochrome are so intimately related to the eventual development of hepatotoxicity.
The fundamental mechanisms of hepatic damage mediated by drugs are regulated by the focal point where this is directed; drugs or their metabolites can have one or several focal points. Some thus attack epithelial cells (acetaminophen), but others attack sinusoid endothelial cells (anaesthetics, alcohol) or biliary epithelial duct cells (chlorpromazine) or generate endothelitis (chemio-therapy). Sometimes damage occurs because the wall itself is destroyed, directly through inducing aptenos making a cell sensitive to antibody-mediated damage, others because they interfere with chemical-chain mediators interfering with a cell’s vital functions, making them fragile and susceptible to damage or because they interfere with metabolic processes, making them accumulate toxic substances which finally destroy hepatocytes.
The main mechanisms “of corruption” generating hepatic damage due to toxicity are:16
- 1.
A drug or its metabolite bind covalently to certain enzymes or intracellular transporters such as nucleotide reverse transcriptase inhibitors, inhibiting mitochondrial DNA production, or inducing intracellular oxidative stress by glutation being consumed or putting an end to a cell’s anti-oxidative machinery. The natural repair process often employs cytokines following such damage, bringing them into contact with labile hepatic cells which destroy them, generating necrosis and inflammation (Figure 2).
- 2.
On other occasions, a drug or its metabolites interfere with the metabolical and energetic machinery existing at canalicular level, which is responsible for secreting bilis. On interrupting its action, it slows down the process of secreting substances which are normally filtered by the bilis and induce cholestasis. A recent finding having transcendental importance has been the fact that, amongst the retained substances, some exercise functions inducing Cytochrome P450, which can increase generating other toxic metabolites, originated by a drug itself or other circulating drugs.
This induction is mediated by orphan transcription and reception factors such as pregnan X (PXR) and constitutive androstan receptors (CAR). This effect can be beneficial as happens with rifampicin activating PXR, in turn mediating Cytochrome CYP 3A4 transcription detoxifying and removing biliary acids and pruriginous substances. This is why this drug has been successfully used in treating some patients suffering from primary biliary cirrhosis.
Establishing the diagnosis: “Association or Guilt”Sometimes it is very easy to establish which drug has been responsible for producing damage in patients having an adverse drug reaction; on other occasions it is very difficult and time-wasting since a patient does not remember exactly what he/she has consumed or a patient is taking several drugs, making identification of which is responsible very difficult. As doctors, we must have very clear tools allowing us to approach the cause, since it is not justimportant for the patient in particular but society in general, including cases of civil and sometimes penal responsibility in which we can become involved.
In this context, it is very important to differentiate between two frequently-confused terms: Association and Causality. A start must be made from the basic principle stating that, “What demonstrates Association is not the same as that demonstrating Causality.”
Many studies Associate an Exposition variable with an Outcome variable17 as happens in analytical, observational Case and Control or Cohort studies. Such association is reported using instruments measuring Indirect Relative Risk (Odds Ratio) or Direct Relative Risk (Risk Ratio) and the usual interpretation of this association is interpreted as being the cause. It is not taken into account that other variables intervening in such association can confound this involvement and that if the are not born in mind they will lead us into making dramatic errors which only show our genuine ignorance in understanding these simple epidemiological concepts. An extremely didactic example has been the association between carrying matches in a pocket and developing lung cancer; this is simple a clear example of association, not of causality, since having matches in the pocket is simply a variable confusing the association between smoking and developing lung cancer. It is thus important to bear in Mind the criteria which must be fulfilled for establishing causality and which can be very useful in certain cases of clinical practice. These are:17
- 1.
Temporal relationship;
- 2.
Strength of the association;
- 3.
Dose relationship/response;
- 4.
Replication of findings;
- 5.
Biological plausibility;
- 6.
Considering alternative explanations;
- 7.
Ceasing being exposed;
- 8.
Association specificity; and
- 9.
Consistency with knowledge.
Several scales have been described and employed from the above list, awarding points which can be used for establishing Causality in diagnosing an adverse hepatic reaction to drugs. Amongst the various scales recommended by their simplicity, coherence and proven validation is Rucam,18 given below.
This scale is easily applicable to any patient in whom the presence of drug-induced hepatic toxicity is suspected. Such diagnosis can be discarded in patients whose point-score may be less than 2, but if it is greater than 8 then the diagnosis is highly accurate.
Specific toxic hepatitis: “a special case in adolescents”There has been the opportunity to attend 57 cases of adverse reaction to drugs during the last 10 years in the Fundación Santa Fe de Bogota, with 10% mortality. The three most important causes in adolescents are: cocaine, Ecstasis and isotretinoin.
Toxic damage caused by cocaine.6,13,19 Consuming cocaine can induce severe pictures of hepatitis in some patients. It is known that the CYP3A4 portion of Cytochrome P450 catalyses cocaine demethylation into norcocaine. This is then oxidized to hydroxinorcocaine which is transformed into a metabolic nitroxide norcocaine free radical reagent mediating hepatotoxicity, since this is then oxidized into ion nitrosonium which has a powerful reaction to glutation, inducing its hepatic depletion. When glutation becomes depleted, this favours free radicals being formed, hepatocytes’ covalent binding to cell wall proteins and lipid peroxidation of the cellular membranes with consequent cellular necrosis.
Even though the presence of hyperthermia and the development of shock can be frequently observed in patients suffering from cocaine poisoning as well as these patients presenting very high aminotransferase levels (almost the same as those seen in patients with ischemic hepatitis); however, ischemia is NOT the primordial factor in developing hepatotoxicity due to cocaine. The damage has rather to do with action damaging hepatic cells mediated by cocaine metabolites originating from CYP3A4 Cytochrome action.
This metabolic fact has therapeutic implications since this enzyme’s activity (as noted in the previous section) can be induced by both rifampicin and some anticonvulsants, consuming alcohol, acetaminophen and barbiturates, thus increasing cocaine’s hepatic toxicity. It should be emphasised that consuming acetaminophen happens most frequently and is most able to increase damage caused by cocaine, since it not only induces CYP3A4 but also diminishes the effective, useful content of intra-hepatic glutation.
This fact must be born in mind when instilling in our patients that if they are going to consume this drug, then they must not just avoid consuming this type of analgesic, but also not submit themselves to prolonged fasts, which can also diminish the intra hepatic glutation level.
Another important aspect is that this enzymatic machinery can be inhibited by the potential protector effect for developing hepatotoxicity induced by erythromycin, ketoconazol, omeprazol and grapefruit juice.
The medical picture observed in patients suffering from cocaine-induced hepatotoxicity is very similar to that induced by acetaminophen poisoning, where necro-inflammatory activity is intense and is frequently associated with developing acute hepatic failure, renal failure, intra-vascular disseminated coagulation and rabdomiolysis. From the histological point of view,20 necrosis with peri-centrally located coagulations, macro vesicular steatosis and slight inflammatory infiltration can be found; changes are more intense in zone 3 of Rappaport’s acino, as is usually seen in en patients suffering from acetaminophen poisoning.
Managing patients suffering from severe cocaine-induced hepatotoxicity is generally done in the Intensive Care Unit, with suitable haemodynamic support usually supplied by this type of Unit associated with haemodialysis (as happened with three of our patients). It is our practice, based on the plausible reasoning of the pathogenesis of the type of damage induced by this toxic substance, to prescribe cystein N-acetyl for such patients infused in 50 mgs per kilogram dose, delivered in 5% Dextrose in an infusion given every 5 hours a day and for at least 5 days. Misoprostol is given in a 200 microgram dose each 8 hours for 10 days.
Damage caused by Ecstasy.6,13,21 This is a meta-amphetamine similar to LSD initially patented as an appetite suppressor, though now widely used as a recreational drug.
This drug has been associated with developing severe hepatic damage, even fulminant failure, requiring hepatic transplant in some cases or leading to death in others. The following can be found amongst those cases reported in the literature: hepatomegally, rash, intense icthericia, marked level of billirubin and disproportional elevation of AST compared to increased ALT, probably reflecting a concomitant alteration of muscular metabolism similar to that reported in cases of malign hyperthermia. This type of toxicity is only managed through haemodynamic support associated with specific managing of complications due to other organs involved becoming compromised. It can be stated as an academic corollary that, “All young people having unclear alterations in their biochemical hepatic and hepatomegally profile quickly suggests the possibility of illicit drugs having been consumed”.
Damage caused by isotretinoin.5,22 This drug is a synthetic retinoid which is very useful in treating cutaneous diseases, especially treating acne. Different to vitamin A, this is a non-predicable toxin, but 10% to 25% of those patients consuming this drug present elevated seric levels of hepatic enzymes. These alterations usually become normalized when the dose is lessened. There are at least 10 cases of severe hepatitis with this drug in the literature; others have been associated with developing chronic fibrosis. It is important to note that this drug’s half-life is 100 days, and it is therefore vital that any patient submitted to being treated with this medication be evaluated very rigorously, at least each 15 days from the doctor’s and paraclinical points of view. It is recommended that this drug be suspended if aminotransferase levels rise above two and a half times the upper limit of the normal range and, if they persist in being elevated, a hepatic biopsy must be done to establish the degree of fibrosis and intensity of the inflammation.
How to act or “What to do”. When faced with hepatotoxicity developing, a physician must act preventatively or curatively. The main action is not to formulate unnecessary drugs; it is estimated that approximately half of the drugs prescribed for patients do not have solid scientific justification for being formulated. If a drug must definitively be used, then in the future knowing the genetic panel will provide us with extremely valuable information for preventing much of the toxicity seen today.
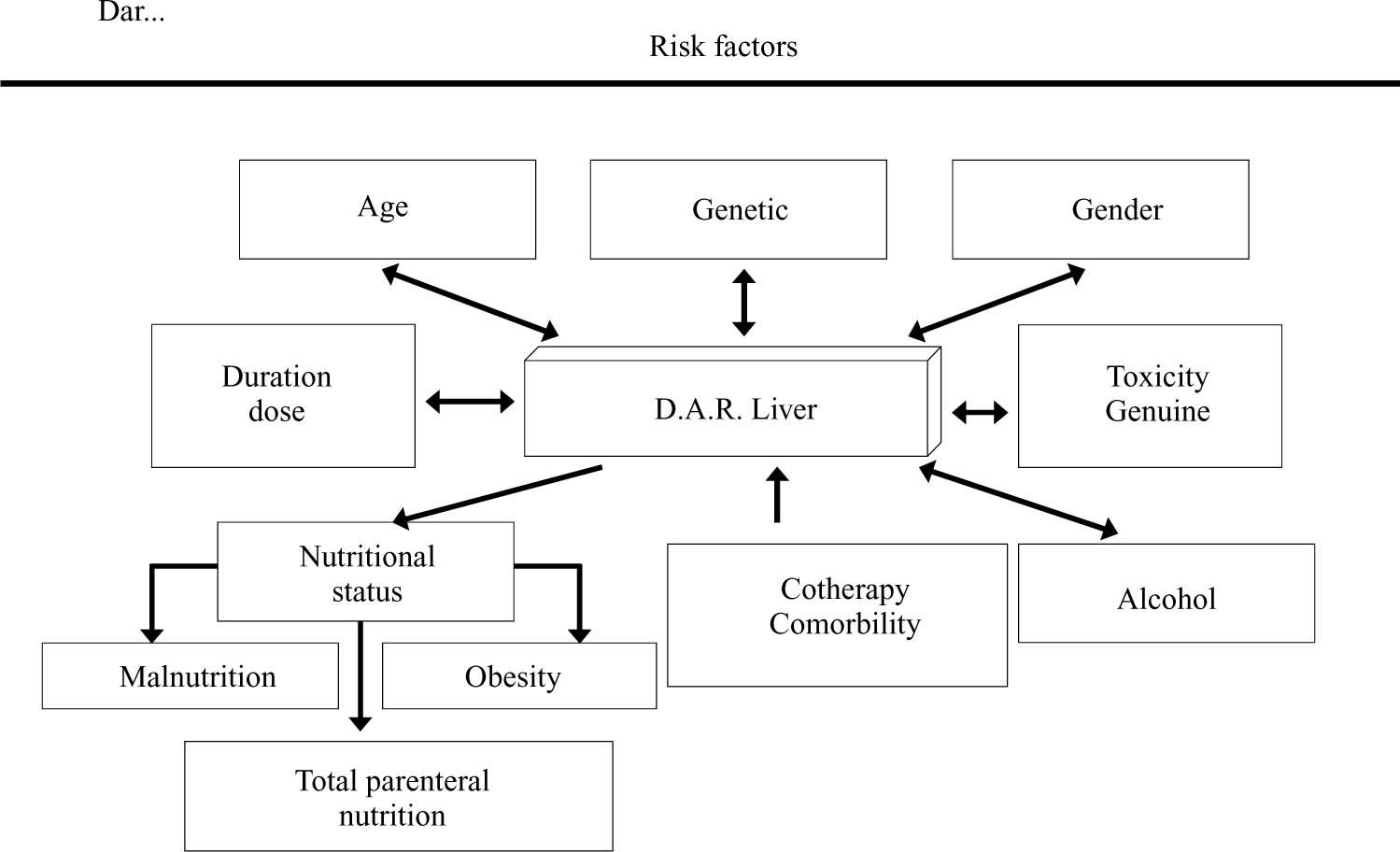
However, not having such information available at the moment, a doctor must make an exhaustive clinical history regarding formulating particular medication. Personal and family antecedents of hepatotoxicity must be especially investigated and risk factors associated with developing hepatotoxicity must be born in mind (a drug’s environmental, morbid and intrinsic ones) to avoid as far as possible their occurrence or at least to detect them as soon as possible (Figure 3).
Once a medication has been initiated which a doctor suspects could be incurring an important risk of hepatic damage, based on the previously evaluated conditions, a patient must be monitored at hepatic enzyme level each 8 days at the beginning and then, following two or three weeks, every 20 to 30 days. The parameters used are the level of hepatic enzymes per enzyme 2.5 to 3 times the normal value meriting greater vigilance, lowering the dose or suspending it, depending on the presence or absence of associated symptoms and a patient’s general clinical condition.