Introduction: The hemostatic system in newborn is a dynamic evolving process health-status dependent. Objective. To explore the changes in coagulation inhibitors and fibrinolytic system in newborn with neonatal cholestasis, according liver damage intensity. Methods: In a cross-sectional design, we studied fibrinolysis and coagulation inhibitor proteins, and serum ferritin (SF) in patients with neonatal cholestasis. We stratified the cases according the results of ALT or AST < 100 UI (group I) and ≥100 U/L (group II). Results: We included 24 newborn, 8 for Group I and 16 cases for group II. We documented statistical differences in ATIII values (43.4 vs 27.4%, p < 0,01), plasmin inhibitor (93.5 vs 63.6%, p < 0.01), SF (649 vs 1410 ug/L, p 0.01). The group II cases showed association with SF values (f 20.6, p < 0.01) and plasmin inhibitor (f 40.4, p < 0.01). AST and ALT were related significantly to ATIII concentrations and SF. Discussion: We documented tendency to prothrombotic state (lower ATIII and greater plasmin inhibitor activity), with low plasminogen related to the intensity of liver dysfunction in neonatal cholestasis. We need to determine the role of iron overload in physiopathology of the disease.
The hemostatic system in fetal life and the newborn period differs broadly from the conditions reported in later age.1 The plasmatic concentration of procoagulant proteins displays different values, related fundamentally with gestational age.2 The newborn habitually expresses prolongation of prothrombin time, activated thromboplastin time, and thrombin time,3,4 with respect to the values of the adult.4 This difference is greater in preterm newborn,5 with lower values of procoagulant proteins (prothrombin, factors V, VII, IX, X) or coagulation inhibitors (ATIII, protein C or protein S). These changes are independent of the perinatal pathology that could happen in this stage of the life.5 In healthy newborn, the delicate changes in the balance of the hemostatic system work acceptably. Nevertheless, in sick newborn, a greater predisposition is observed on clinical expression of the dysfunction of the hemostatic system, expressing by hemorrhage, thrombosis or both. In children or adult with chronic hepatic disease, clinical and biochemical alterations of hemostasis are known widely.6,7 These alterations frequently comprise the clinical expression of the disease that complicate the evolution of the patient.
The transitory neonatal cholestasis (TNC), is a condition of acquired etiology and spontaneous resolution,8 with alterations in the biliary flow at level of the hepatocyte or biliary conducts; with biliary pigment deposit in hepatocytes.9 These newborns had prolonged jaundice between the second and tenth weeks of life.10 Characteristically in TNC the increase conjugated fraction of bilirrubin exceeds 2.5 mg/dL, that constitutes at least 20% of the bilirrubin11 and it is accompanied by data of hepatic failure of variable degree.8 The causes of TNC include events generally associate with perinatal period, asphyxia, 12 preterm infant,13 sepsis,14 parenteral nutrition or intermittent mechanical ventilation.15 Nevertheless, we do not have information on the intensity of the alterations of hemostatic system in TNC and if some specific biochemical pattern exists or if these alterations are own of the neonatal condition or they are related with the hepatic failure.
The objective of this report is to present the results of the changes of inhibitors of the coagulation and fibrinolytic system in newborn with acquired cholestasis syndrome, with the intention to contribute at clinical knowledge of the physiopathology of alterations of hemostasia with neonatal cholestasis.
Material and methodsWith a cross-sectional design, we selected consecutively newborns with TNC. They were stratified according the intensity of hepatic damage in two groups: The group I, cases with values of aspartate aminotransferase (AST), alanine aminotransferase (ALT) or both, ≥ 100 UI/L. Group II with values 3 100 UI/L.8,13,16 The TNC diagnosis was made at second week of life by increase of serum bilirrubin conjugated > 2.5 mg/dL. In all cases an ultrasonographic study carries out intentionally to explore the hepatic area and biliary tree to discard associate structural malformations to cholestasis of congenital etiology. We excluded all newborn with serologic reactivity to hepatitis B antigen surface or antibody against the hepatitis C virus. In first two days after establishing the cholestasis diagnosis, we collected 250 uL of venous blood in a tube with EDTA and 1.8 mL in another tube with sodium citrate 3.2%. Immediately we determined the platelet count in an electronic particle counter (ABX MICROS 60-OT). All plasma samples were stored at < 70 degrees Celsius until further processing. We determined D-dimer concentration (DD), by agglutination technique of latex particles covered with a monoclonal antibody against fibrin domain D (Fibrinosticon, Organon Teknika). We measured with chromogenic techniques, antithrombin III (ATIII, abnormal value < 30%), and plasminogen (Pg abnormal value < 70%), and plasmin inhibitor (InhPl abnormal value > 120%). The ELISA technique was used for quantification of plasminogen activator inhibitor type 1 antigen (Asserachrom PAI-1, normal values < 43 ng/mL), protein C (PC, Thrombonostika C protein, Organon Teknika, with abnormal values < 0.30 UI/mL). As well for determination of total protein S and free protein S (TPS and FPS, with abnormal value < 66 and < 55%, respectively), FPS was determined after polyethylenglycol treatment of plasma. The serum ferritin (SF, abnormal value > 1,000 ug/L) was processed by ELISA with double antibody technique (Behring, Enzygnost). ALT and AST expressed in IU/L were determined by enzymatic methods. Finally, we collected the information about the postnatal age in which cholestasis diagnosis was made, number of days in parenteral nutrition or intermittent mechanical ventilation.
According of values distribution (Kolmogorov-Smirnov), median and percentile range (25-75) were described. In variables with normal distribution, average and 95% of confidence interval were obtained. In order to compare the difference between variables of study between both groups, median difference test for two independent samples was applied. In relation between dependent variable (abnormal hepatic enzymes) and independent variables (coagulation and fibrinolytic tests), we applied a stepwise analysis regression. The significance level was < 0.05.
ResultsWe included 24 newborn with TNC diagnosis, eight cases in group I (AST and ALT, median 47, quartile 29-72 IU/L), and 16 newborns in group II (AST/ALT median 133, quartile 102-224). When comparing demographic data between groups. We do not observed statistically significant differences in gestational age (35.6 vs 36.3 weeks, respectively), proportion of preterm infants (0.75 vs 0.68). As well as days of total parenteral nutrition (36 vs 43), age of diagnosis of cholestasis (33 vs 26), days in intermittent mechanical ventilation (14 vs 20 days), serum concentrations of conjugated bilirrubin (7.3 vs 9.2 mg/dL), or number of transfusions (Table I). There were statistically significant results in serum ferritin (649 vs 1410 ug/L, Mann-Whitney test, p 0.01), as well proportion of cases with iron overload (0.25 vs 0.94, p 0.01).
Comparation between general variables of study groups.
| Variable | Group I (n 8) | Group II (n 16) |
|---|---|---|
| Gestational age (weeks) † | 35.6 (32.6-39.0) | 36.3 (34.6-38.0) |
| Preterm neonates (n) | 6 (0.75) | 11 (0.68) |
| Total parenteral nutrition (days) † | 36 (19-54) | 43 (28-58) |
| Age of cholestasis diagnosis (days)† | 33 (14-52) | 26 (17-35) |
| Intermittent mechanical ventilation (days) 14 | 14 (1-23) | 20 (5-29) |
| Serum ferritin (μg/L) * | 649 (327-1012) | 1410 (1085-2246) |
| Direct serum bilirrubin (mg/dL) | 7.3 (3.5-9.9) | 9.2 (7.4-14.1) |
| Transfusional events (n/proportion) | 7 (0.87) | 9 (0.56) |
| Iron overload (n/proportion with SF > 100 μg/L) * | 2 (0.25) | 15 (0.94) |
In comparison of difference of averages between hemostasia proteins, according to training groups (Table II). We don observed statistically significant differences in platelet count (204 vs 169 xmm3), proportion of cases with thrombocytopenia (0.25 vs 0.56), protein C concentration (0.27 vs 0.25 UI). As well as cases proportion with PC < 0.30 IU/mL (0.62 vs 0.75), total protein S (49.0 vs 52.0%), free S protein (33.5 vs 38.1%), D-dimer (250 vs 500 ug/L), plasminogen (42.2 vs 43.2%), and PAI (116 vs 145 ng/mL). We observed differences in ATIII (43.4 vs 27.4%, p < 0.01) and in plasmin inhibitor (93.5 vs 63.6%, p < 0.01). In order to evaluate if diagnosis age has any influence in ATIII values. We applied mean difference for independent samples (Kruskall-Wallis test), without statistically significant differences (data no showed).
Comparation between hemostatic values in both studies groups.
| Variable | Group I (n 8) | Group II (n 16) |
|---|---|---|
| Platelets (x103) † | 204 (109-299) | 169 (119-220) |
| Thrombocytopenia (n/proportion) | 2 (0.25) | 9 (0.56) |
| Protein C (UI) | 0.27 (0.22-0.53) | 0.25 (0.20-0.30) |
| Protein C < 0.30 UI (n/proportion) | 5 (0.62) | 12 (0.75) |
| Protein S total (%) | 49.0 (39.5-84.0) | 52.0 (42.5-61.5) |
| Total protein S < 40 % (n/proportion) 2 (0.25) | 2 (0.25) | 3 (0.19) |
| Free Protein S (%)t | 33.5 (28.1-38.8) | 38.1 (33.0-43.3) |
| D-dimmer (Ug/L) | 250 (0-500) | 500 (0-650) |
| D-dimmer high (> 1000 ug/L) | 1 (0.12) | 3 (0.18) |
| Antithrombin III † | 43.4 (33.0-53.8) | 27.4 (20.9-33.9) |
| Low ATIII (n/proportion) | 4 (0.50) | 13 (0.81) |
| Plasminogen (%) † | 42.2 (26.0-58.3) | 43.2 (34.4-52.1) |
| Plasmin inhibitor (%) *† | 93.5 (77.5-109.5) | 63.6 (55.0-72.1) |
| PAI (AU/mL) | 116 (64-194) | 145 (84-205) |
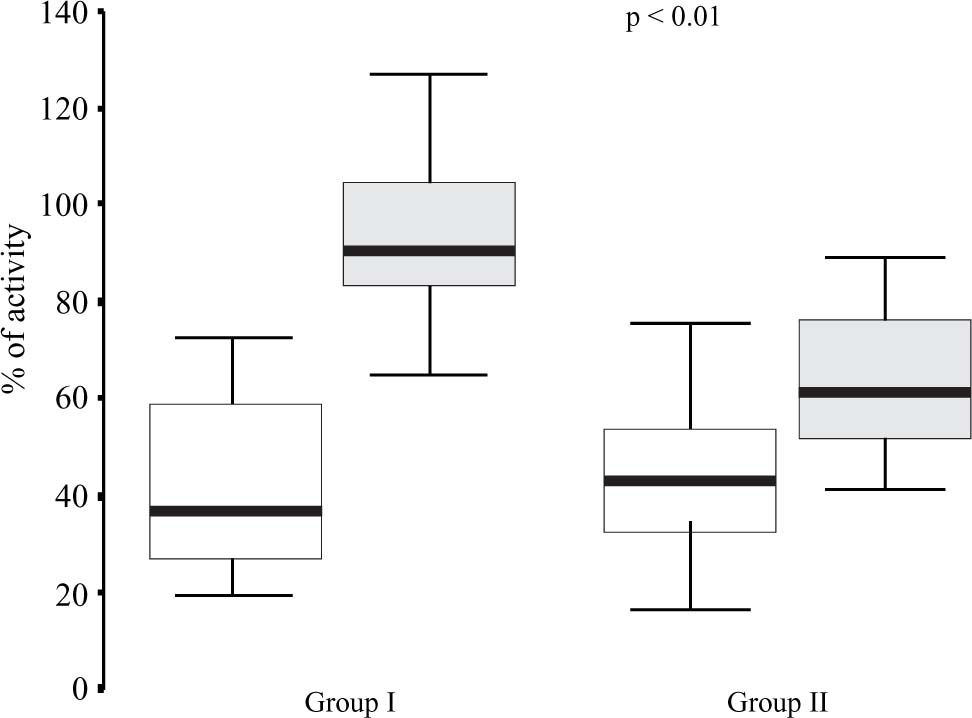
With the intention to compare the differences of the changes in median values of plasminogen and plasmin inhibitor, according to study groups. We observed statistically significant differences in plasmatic concentrations and plasmin inhibitor (p <0.01), but without significant changes in plasminogen (Figure 1).
We observed statistical association between both groups by stepwise regression analysis, in SF concentrations (f 20.6, p < 0.01) and plasmin inhibitor (f 40.4, p < 0.01), as well AST and ALT concentrations were related significantly to ATIII values (f 10.7 and 4.6, p < 0.01 and 0.04, respectively) and SF values (f 28.1 and 26.2, p < 0.01) (Table III).
Stepwise analysis regression of liver enzymes, serum ferritin, and hemostatic values.
| Dependent Variable | Independent Variable | Value of f | Value of t |
|---|---|---|---|
| Aspartato aminotransferase | Antithrombin III * | 10.7 | -3.2 |
| Serum ferritin * | 28.1 | 5.3 | |
| Alanine aminotransferase | Antithrombin III ** | 4.6 | -2.1 |
| Serum ferritin * | 26.1 | -5.1 | |
| Both group (I and II) | Serum ferritin * | 20.6 | 4.5 |
| Plasmin Inhibitor * | 40.4 | -6.3 |
The antigen concentration and functional activity of coagulation and fibrinolytic proteins are used to evaluate hemorrhagic or thrombotic disorders in newborn period. 1,2 Delicate balance of neonatal hemostasia summary of the following as: A). The procoagulant proteins show a global decreased of activity and function (v gr. vitamin-K dependent proteins or contact phase system), but with normal values of FVIII and FV and fibrinogen showed similar concentrations to adult, but their function is diminished. These results explain the habitual prolongation on the coagulation tests (prothrombin time, activated partial thromboplastin time, and thrombin time), in the neonatal period.17 B) The coagulation inhibitors are diminished in concentration and function (PS, PC, and ATIII). C) Fibrinolytic system showed a decreased in antigen concentration and protein function of plasminogen (fetal plasminogen) and tissue plasminogen activator (t-PA). D) Anti-fibrinolytic system show similar values to adults (plasmin inhibitor, PAI).
The changes in hemostatic system in sick newborn have been less characterized with absence of specific patterns of hemostatic response. This is due to three fundamental conditions: technical difficulty to study newborn infant, the changes related to gestational age (vitamin-K dependent factors) and effect of maternal health like preeclampsia or drug ingestion.1
In other ages of life, extensive information is had on biochemical alterations and clinical expression of hemostatic defects in patients with acute18 or chronic liver disease. 19,20 Nevertheless, in newborn with liver dysfunction, have sufficiently changes in hemostasia system that finally can be expressed clinically like hemorrhage, thrombosis or both of them. The problems added in newborn with liver dysfunction, along with changes of their gestational age, add some transitory and innocuous diseases as the physiologic jaundice or related to the direct morbidity of the neonatal period as asphyxia,12 preterm birth,13,21 distress respiratory12,22 or neonatal sepsis.21
In neonatal cholestasis, in presence of liver inflammation, we documented the diminution of natural inhibitors of coagulation, as antithrombin III, but without difference in protein C, protein S, total or free form. The values of protein S (total or free) and the PC reported are similar to the described by another authors.2,23 This is a reflection of global alteration of liver dysfunction in protein synthesis; this is the case of ATIII, with biochemical evidence of greater prothrombotic risk.24,25
The components of fibrinolytic system are present since birth, but plasminogen is diminished in concentration and function in near 50% of adult values.26,27 In present reports, we documented the significant diminution of plasmin inhibitor in patients with greater liver affectation that in controls (63.6 vs 93.5), but without statistical difference in plasminogen concentrations, between study groups. The increase of plasmin inhibitor could mean the intensity of alteration of the hepatic dysfunction in cases with neonatal cholestasis.14 It is interesting to observe that PAI is over values referred for adult.4 These changes, along with the increase of the tissue factor, are a reflection of the increase of interleukins, in special IL-6 that occurred in neonates with hepatic disease.27 Like the changes observed with inhibitors of the coagulation, the alterations of the fibrinolytic system are interpreted as a prothrombotic state of the newborn infant with cholestasis.