A 79-year-old man was admitted to our hospital because of increased hepatobiliary enzyme levels. Dynamic computed tomography and magnetic resonance imaging showed a liver tumor measuring 60mm containing fat foci at the cranial aspect of the tumor. We diagnosed the patient with hypovascular hepatocellular carcinoma (HCC) and fat deposition, and performed a caudate lobe resection. Pathology examination revealed two intermingled components: moderately differentiated HCC with fat deposition and neuroendocrine carcinoma (NEC). Primary combined NEC and HCC is extremely rare. To our knowledge, this is the first report of combined NEC and HCC including a fat component. HCC is the most common primary hepatic malignancy with fat. HCC might include fat, even if HCC coexists with another type of cancer. The imaging characteristics of and HCC with another type of cancer vary depending on the amount of each component. We should not simply diagnose such tumors as HCC, but think about the possibilities of HCC with another type of cancer, because there is a fat component.
Both primary hepatic neuroendocrine tumors (NET) and neuroendocrine carcinoma (NEC) are rare [1], and primary combined NEC and hepatocellular carcinoma (HCC) is especially rare. Little has been reported on primary combined NEC and HCC because of the rarity. It is difficult to diagnose hepatic NET without histopathologic examination because the computed tomography (CT) and magnetic resonance imaging (MRI) features vary [2,3]. To our knowledge, there are no reports of combined HCC and NEC including a fat component. Here we report a patient having an extremely rare primary combined NEC and HCC with unique imaging characteristics showing that the tumor included definite fat deposits.
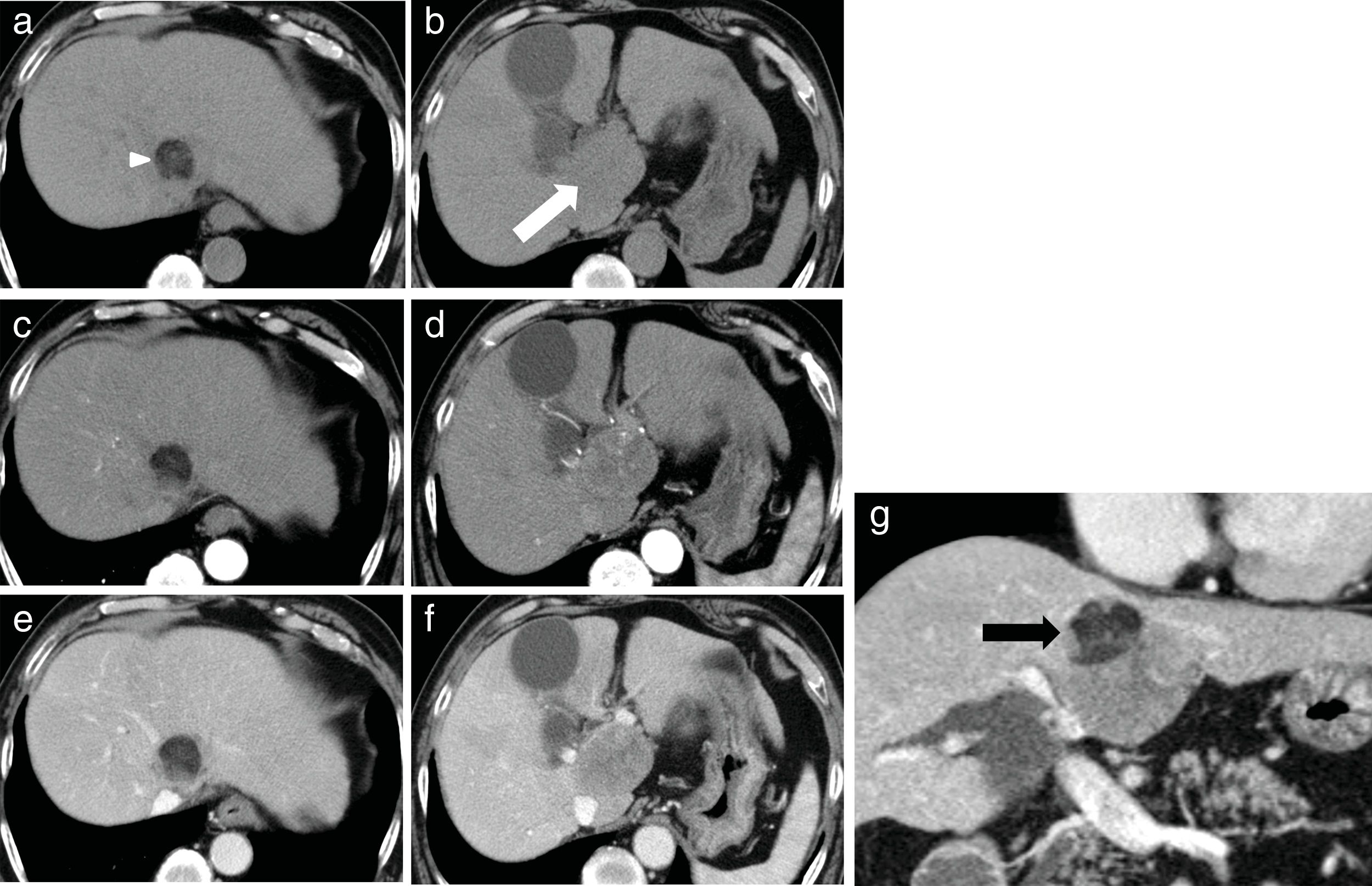
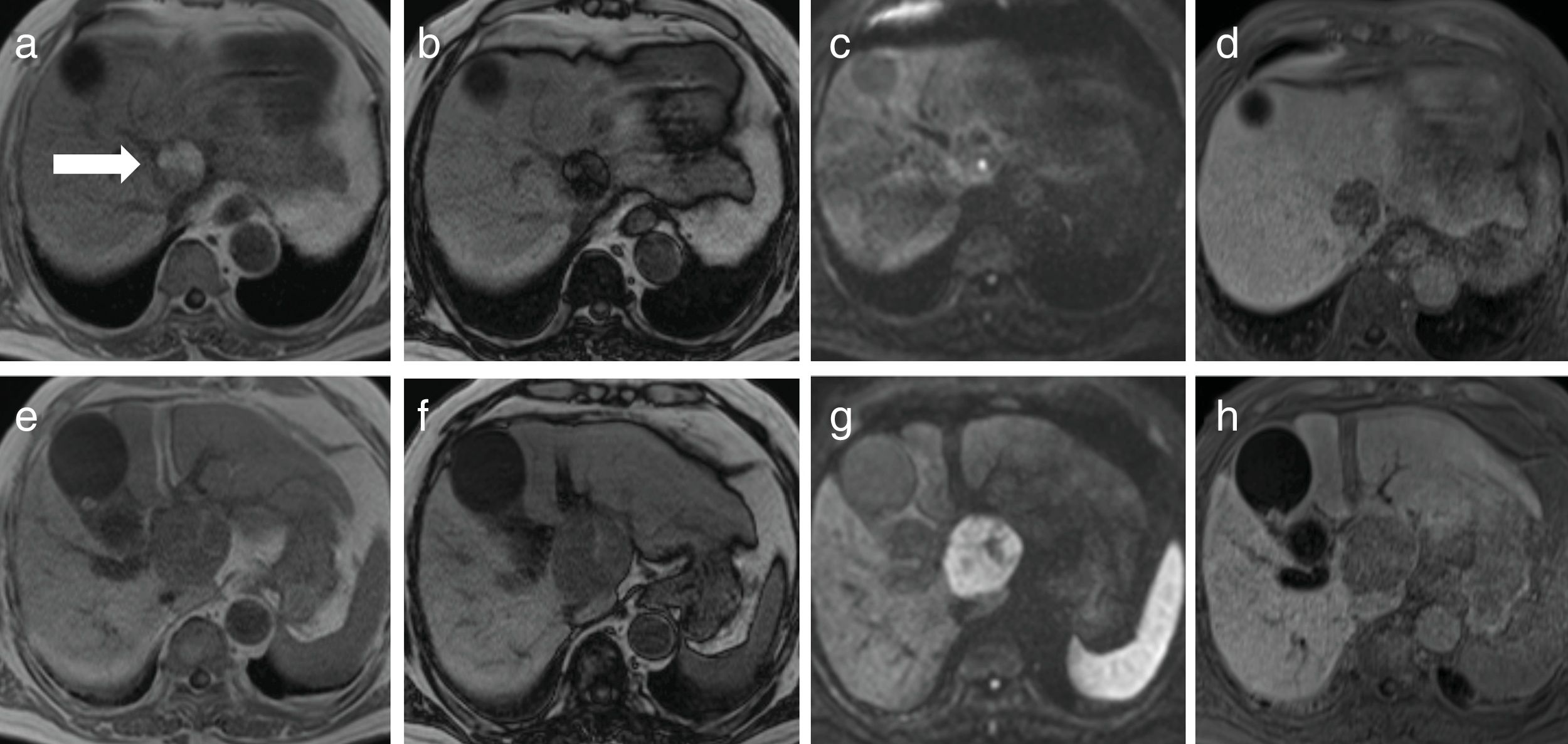
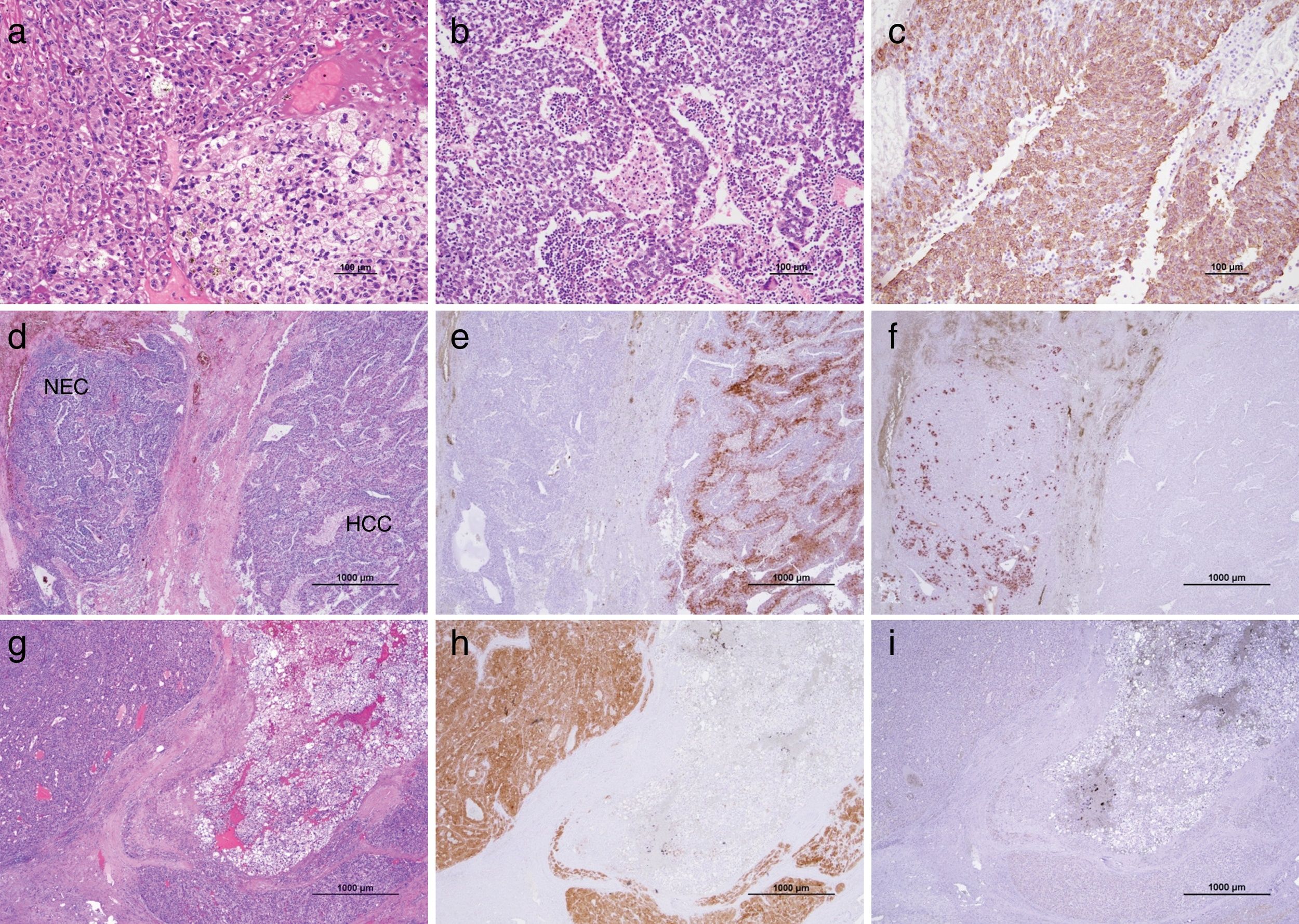
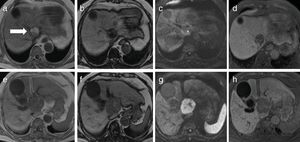
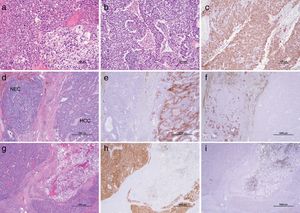
2CaseA 79-year-old man presented with increased hepatobiliary enzyme levels at a periodic laboratory examination by his primary doctor and was admitted to our hospital with no complaints in 2017. He was on regular medication for diabetes mellitus, hyperlipidemia, hypertension, angina pectoris, and atrial fibrillation. Laboratory examination results revealed an aspartate aminotransferase level of 66IU/L, alanine aminotransferase level of 34IU/L, alkaline phosphatase level of 1159IU/L, a gamma-glutamyl-transpeptidase level of 597IU/L, and total bilirubin level of 2.5mg/dL. Serologic markers for hepatitis B and C viruses were all negative. Among tumor markers, the alpha fetoprotein level was 3231.8ng/mL. Ultrasonography (US) examination revealed an enlarged gall bladder and dilation of the common bile duct. In addition, a hypoechoic liver tumor measuring 60mm containing a partly hyperechoic lesion was detected in the caudate lobe. Arterial phase CT showed an iso-enhanced tumor in segment 1 (Fig. 1). Portal phase CT of the tumor showed low attenuation. The tumor contained a very low-density lesion at the cranial aspect of the tumor. The area showed no enhancement in all-phase CT, and was considered to be foci of fat. There was no other lesion outside the liver tumor. Gadolinium-ethoxybenzyl-diethylenetriamine-enhanced MRI of the tumor showed iso-intensity in the arterial phase with subsequent washout in the portal phase. The tumor was detected as a defect in the hepatobiliary phase. The cranial aspect of the tumor had a loss of signal intensity on chemical shift imaging, indicating the presence of fat (Fig. 2). The tumor was thus preoperatively diagnosed as hypovascular HCC with fat deposits, which had compression to biliary system. We performed a caudate lobe resection. Pathology examination of the resected specimen revealed two intermingled components: a moderately differentiated HCC component with a trabecular pattern and fat deposition, and a NEC component comprising small tumor cells with a high nuclear to cytoplasmic ratio and fine chromatin (Fig. 3). Immunohistology revealed that the NEC component strongly expressed chromogranin A, and was negative for arginase-1. On the other hand, the tumor cells of the HCC component were positive for arginase-1 and negative for chromogranin A There were foci of fat only near the HCC component. There were no tumor cells positive for both arginase-1 and chromogranin A. Thus, the patient was diagnosed with primary combined NEC and HCC. The tumor had no invasion to bile duct.
Findings of dynamic computed tomography. (a, b) Plain phase showed a 60-mm round tumor in segment 1. The majority of the tumor exhibited slightly low density (white arrow). It contained a very low density lesion, indicating fat deposits, only at the cranial aspect of the tumor (white arrow head). (c, d) The arterial phase showed a round tumor with iso-enhancement. (e, f) The late phase showed hypo-enhancement. The fat deposits at the cranial aspect were avascular in all phases. (g) Coronal view of the late phase showed that the low attenuation round tumor included very low density foci only on the right side of the cranial aspect of the tumor.
Findings of gadolinium-ethoxybenzyl-diethylenetriamine enhanced magnetic resonance imaging. (a) In-phase T1-weighted image of cranial aspect of the tumor showed a hyperintense lesion. (b) Out-phase T1-weighted image of the same slice showed a signal decrease in the lesion. (c) Diffusion-weighted image of the same slice showed iso-intensity. (e) In-phase T1-weighted image of caudal area of the tumor showed a hypointense lesion. (f) Out-phase T1-weighted image of the same slice showed no signal decrease. (g) Diffusion-weighted image of the same slice showed marked hyper-intensity. (d, h) The tumor showed low intensity in the hepatobiliary phase.
Microscopic findings. The resected tumor comprised two components. (a) One was the moderately differentiated hepatocellular carcinoma (HCC) component with a trabecular pattern and fat deposition. (b) The other was a neuroendocrine carcinoma (NEC) component comprising small tumor cells with a high nuclear to cytoplasmic ratio. (c) NEC is positive for chromogranin A. (d) Both components were intermingled with each other. (e) The HCC-dominant area was positive for arginase-1. (f) The NEC-dominant area was positive for chromogranin A. There were no tumor cells positive for both arginase-1 and chromogranin A. (g) HCC with fat deposits was present at the cranial aspect of the tumor, and were positive for arginase-1 (h) and negative for chromogranin A (i).
The patient had a good postoperative course, and was discharged 2 weeks after the operation. Four months later, however, the patient died due to rapid tumor recurrence.
3DiscussionThe liver is a common site of NET metastases from other origins, but primary hepatic NEC is extremely rare. NETs commonly derive from cells of the neuroendocrine system and are mostly observed in the respiratory, gastrointestinal tract, and pancreas [1,4]. NETs sometimes accompany other histologic components, most of which are adenocarcinomas. Those NETs are defined as mixed adenoneuroendocrine carcinomas (MANEC) in the 2010 WHO criteria [5]. The NEC component of biliary MANEC is considered to originate from adenocarcinoma cells [6]. On the other hand, HCC with a NEC component is extremely rare, and the NEC component of combined NEC and HCC may derive from HCC cells [1,7]. Imaging characteristics of pure hepatic NEC are not fully clarified, but might have a varied pattern [2]. We speculate that the imaging characteristics of combined NEC and HCC also have a varied pattern and depend on the amount of each component.
In the previous report, the most effective therapy for primary hepatic NEC is hepatectomy. Other available treatments including chemotherapy, interferon, and targeted therapies also have been mentioned [8]. The survival rate is satisfactory in spite of recurrence; the 5-year recurrence rate is 18%, and the 5-year survival rate is 74–78%. Little has been reported on primary combined NEC and HCC because of the rarity. Although some reports of patients with combined NEC and HCC showed longer survival than pure NEC, there are some reports with an extremely poor prognosis like an our case.
The presence of fat in a liver lesion on imaging is a highly useful diagnostic feature. The fat deposits are observed as high echoic areas in US, less attenuating areas in CT, and chemical shifts in MRI [9]. It is relatively easy to catch these findings from the various imaging modalities. Liver lesions that contain fat vary, and include HCC, regenerative nodules, hepatocellular adenoma, nodular steatosis, and atypical focal nodular hyperplasia [9–12]. They are diagnosed based on some additional findings, such as vascularity, shape, back ground liver condition, and some tumor markers. HCC is the most common primary hepatic malignant neoplasm with a fat component. Fatty changes can be seen in 20–35% of small HCCs [9,11]. On the other hand, combined hepatocellular-cholangiocarcinoma includes a fat component compared to mass-forming intra-hepatic cholangiocarcinoma [13]. This means that HCC might include a fat component, even if HCC coexists with another type of cancer. Thus, we should not simply diagnose such tumors as HCC, but think about the possibilities of HCC with another type of cancer, because there is a fat component.
To our knowledge, there are no reports of combined HCC and NEC including a fat component, and this is the first report. Several combined NEC and HCC cases have been reported to date and were classified into three types, i.e., transitional, intermediate, and separate types [1,7]. The present case was classified as the transitional type according to the histologic and immunohistochemical features. In our case, the fat lesion was included only in the HCC component. Thus, HCC including a fat component intermingled with a NEC component.
In conclusion, combined NEC and HCC are very rare, and combined NEC and HCC with a fat component are even rarer. The imaging characteristics of and HCC with another type of cancer, such as NEC and HCC in this case, vary depending on the amount of each component. Combined HCC and another type of cancer should be included in the differential diagnosis of an unknown liver tumor with fat.AbbreviationsCT
computed tomography
HCChepatocellular carcinoma
MANECmixed adenoneuroendocrine carcinomas
MRImagnetic resonance imaging
NECneuroendocrine carcinoma
NETneuroendocrine tumors
USultrasonography
Grant supportThe authors have no grant support.
Conflict of interestNone.